
- Home
- News
- Analysis
- States
- Perspective
- Videos
- Education
- Entertainment
- Elections
- Sports
- Features
- Health
- Budget 2024-25
- Business
- Series
- Bishnoi's Men
- NEET TANGLE
- Economy Series
- Earth Day
- Kashmir’s Frozen Turbulence
- India@75
- The legend of Ramjanmabhoomi
- Liberalisation@30
- How to tame a dragon
- Celebrating biodiversity
- Farm Matters
- 50 days of solitude
- Bringing Migrants Home
- Budget 2020
- Jharkhand Votes
- The Federal Investigates
- The Federal Impact
- Vanishing Sand
- Gandhi @ 150
- Andhra Today
- Field report
- Operation Gulmarg
- Pandemic @1 Mn in India
- The Federal Year-End
- The Zero Year
- Premium
- Science
- Brand studio
- Newsletter
- Home
- NewsNews
- Analysis
- StatesStates
- PerspectivePerspective
- VideosVideos
- Entertainment
- ElectionsElections
- Sports
- Features
- BusinessBusiness
- Premium
- Loading...
Premium - India-Canada ties
Mars rover: It’s finally time for Nasa's Perseverance to pay off
NASA’s latest rover Perseverance will be launched towards Mars on July 30, 4:50 pm IST (4:50 am PDT) from Space Launch Complex 41 in Florida.

In what appears to be a page straight out of the Hollywood blockbuster The Martian, in a year from now, a hi-tech device will be pulling oxygen out of the thin, carbon dioxide-rich Martian atmosphere. MOXIE—short for Mars OXygen In-situ Resource Utilisation Experiment—will generate a modest amount of oxygen from carbon dioxide through the process of electrolysis. Perched aboard...
In what appears to be a page straight out of the Hollywood blockbuster The Martian, in a year from now, a hi-tech device will be pulling oxygen out of the thin, carbon dioxide-rich Martian atmosphere. MOXIE—short for Mars OXygen In-situ Resource Utilisation Experiment—will generate a modest amount of oxygen from carbon dioxide through the process of electrolysis.
Perched aboard the Perseverance Rover, this instrument, along with several others, will be launched towards Mars on July 30, 4:50 pm IST (4:50 am PDT) from Space Launch Complex 41 at Cape Canaveral Air Force Station in Florida.
Hurled towards Mars by the United Launch Alliance Atlas V rocket, after a torturous journey of 204 days, the spacecraft will touch down in Jezero crater on Mars on February 18, 2021.
Fitted with a suite of hi-tech instruments, 23 cameras, two microphones, a laser to vaporise rock and a special rotary percussive drill to gather rock cores samples, NASA’s latest rover will explore the Red Planet like never before. The objective is to shed light on whether life ever arose on Mars and also characterise its climate and geology.
All this has become routine. But what will set apart this mission from the rest are the baby steps towards the human exploration of Mars in future.
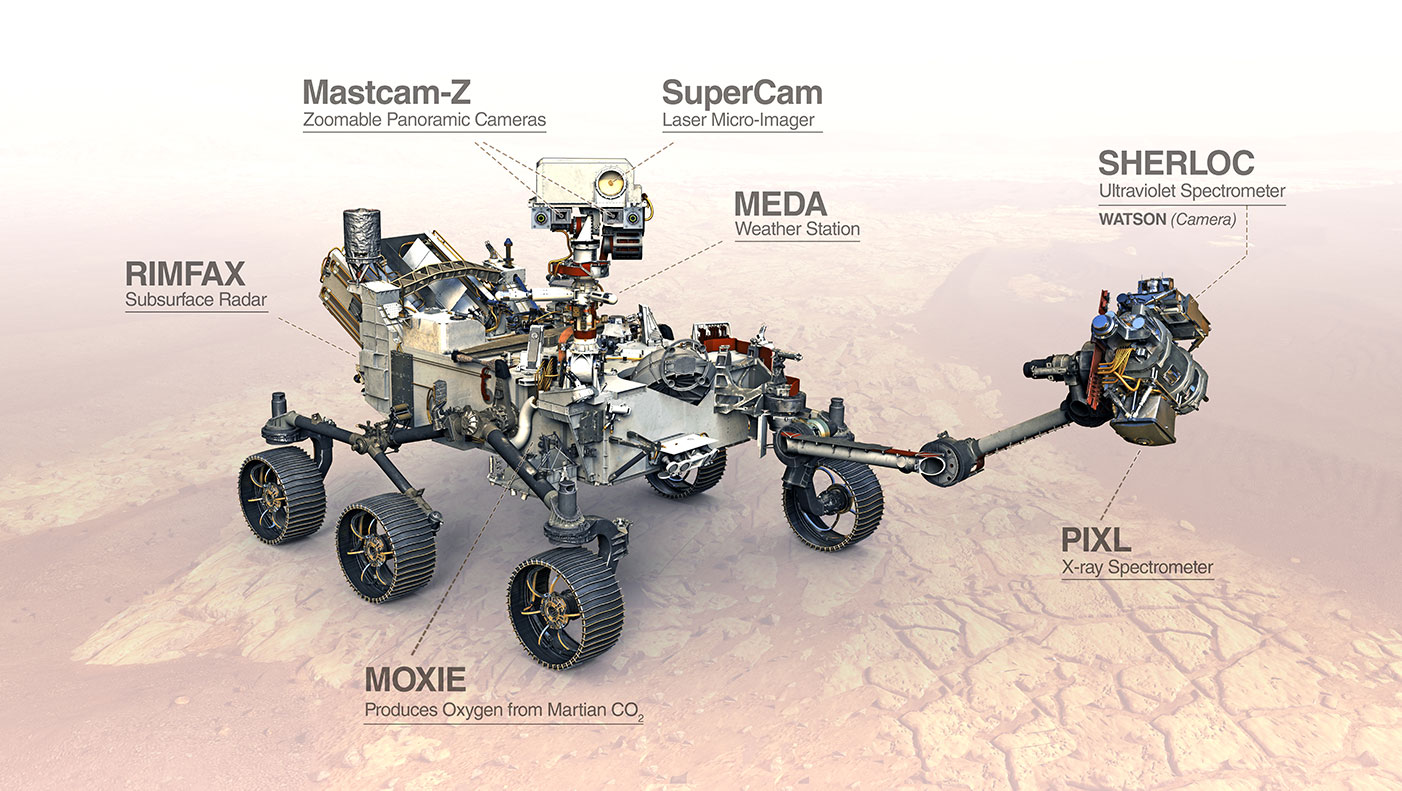
The oxygen-making factory, MOXIE, demonstrates in-situ resource generation, rather than lugging every resource from Earth. A small compact toy-like helicopter named Ingenuity, weighing 1.8 kg and having a flight range of 300 meters will be the first-ever aerial flight on another world.
The mission will also drill core samples and preserve them in hermetically sealed containers, until a future mission retrieves the samples and brings them back to Earth.
Artificial tree
A nasty surprise will await us if we make a journey back in time in a time machine to Earth’s distant past, say, 2.4 billion years ago. Like a fish out of water, we would be gasping for breath. Yes, the atmosphere of early Earth was devoid of oxygen. Only with the evolution of photosynthesising organisms such as cyanobacteria, the oxygen content of Earth saw an increase.
Using sunlight, these tiny organisms converted water and carbon dioxide into sugars and in the process released oxygen as a by-product. Until then, the life forms on Earth relied on thermal energy from ocean vents and was limited only to niche spaces.
The first sparks of photosynthesis spread like wildfire and the whole Earth was covered with carbon dioxide-inhaling, oxygen-exhaling creatures, ancestors to plants and trees. A revolution, called the Great Oxidation Event (GOE), ensued. From practically no oxygen in the atmosphere, molecular oxygen accumulated reaching the current levels of 21 per cent.
With no trees, plants and vegetation, the Martian atmosphere that is composed of 95.32 per cent carbon dioxide is comparable to the early Earth. With only trace amounts of oxygen and 100 times thinner atmosphere Mars, least to say, is harsh and uninviting.
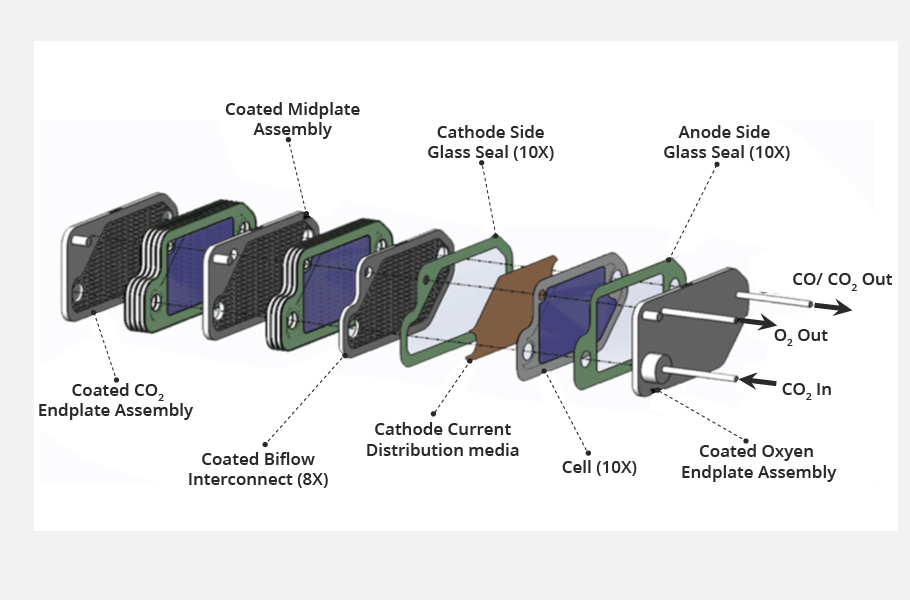
Just like the tiny blue, green cyanobacteria, a Solid Oxide Electrolysis Cell (SOEC) onboard the Perseverance Rover will breathe in carbon dioxide and breath out a tiny amount of oxygen.
Planned as a technology demonstration project, the experiment is to test the novel technology to generate breathable oxygen and liquid oxygen fuel for space missions on the surface of Mars. Perhaps one day in the distant future, the same technology could make the whole planet habitable by enriching the atmosphere with oxygen.
Solid oxide electrolysis cell
The mechanism by which MOXIE works is akin to the hydrolysis of water we are familiar with in our high school chemistry. The electrolysis cell uses electricity to split water molecules (H2O) into hydrogen (H2) and oxygen (O2), which collects at the negatively charged electrode (cathode) and positively charged electrode (anode) respectively.
Likewise, MOXIE will use electricity to break apart carbon dioxide molecules into carbon monoxide and oxygen.
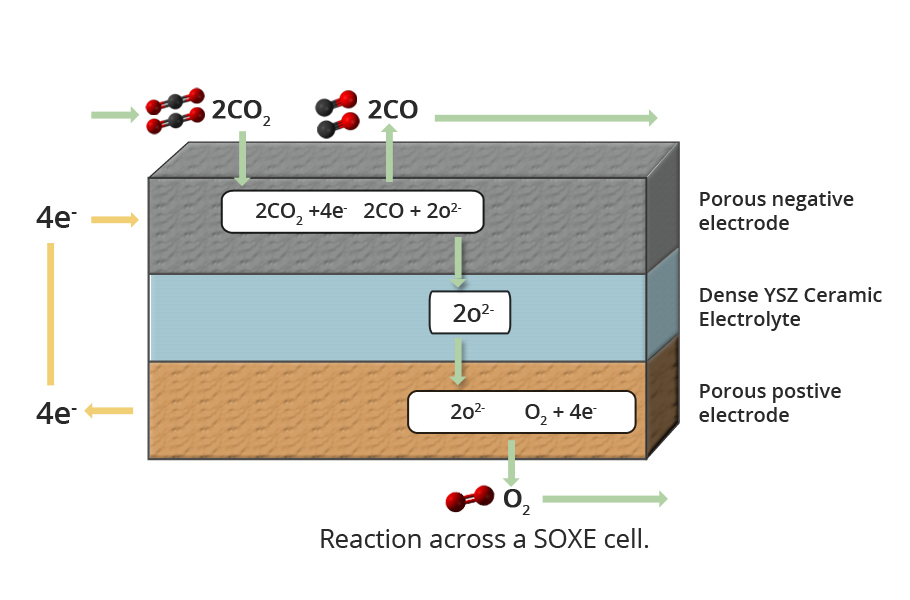
The basic structure of MOXIE consists of a ceramic solid-state electrolyte sandwiched between a metal oxide cathode and anode.
When carbon dioxide is passed over a metal-oxide electrode at high temperatures (800–900°C), using the electrons provided by the cathode, it splits into carbon monoxide and oxygen anion. The oxygen ion enters the pores of the ceramic electrolyte and migrates towards the anode due to the electric field. The oxygen atoms combine at the anode into oxygen molecules and, in the process, liberates the electrons. During electrolysis, carbon monoxide accumulates at cathode and oxygen molecules at the anode.
The process is precarious. The nudge to break the carbon dioxide must be calibrated to make sure that the carbon dioxide fractures into carbon monoxide and oxygen and not carbon and oxygen. The build-up of the carbon is a hindrance and could destroy the system.
Technology
The whole system comprises three main components. A compressor collects and concentrates Martian air to about the density of air at Earth’s surface. The electrolysis cell electrochemically splits the carbon dioxide into oxygen and carbon monoxide.
Lastly, the sensors, particularly in the exhaust instrument, monitor the purity of oxygen being vented back out to the Mars atmosphere along with the carbon monoxide and other exhaust products.
Electricity is needed for both heating up the cathode and also supplying voltage for the electrolysis. Multi-Mission Radioisotope Thermoelectric Generator (MMRTG), a nuclear battery, supplies the required power.
The heart of the MMRTG isnis radioactive plutonium-238. As plutonium-238 naturally decays, it gives off heat. An array of thermocouples to convert the heat released by the decay into electricity by the Seebeck effect.
Using 300 watts of power, and operating at 800 degrees Celsius, MOXIE will produce about 10 grams of oxygen per hour. For comparison, an average adult needs about 20 g of oxygen per hour to breathe. The equipment will be operated for about two hours, in which one hour of effective oxygen production will be carried out per experiment. The experiment will be carried out intermittently throughout the mission.
Human mission to Mars
MOXIE is the size of a car battery. Future oxygen generators that support human missions to Mars must be about 100 times larger. By using multiple electrolysis cells in a single system, the yield of the oxygen can be increased to the desired level.
In addition to production on a larger scale, for life-sustaining activities for human travellers, oxygen is also needed for the rocket fuel for a return trip to Earth. To launch off of Mars, the return rockets will need about 33 to 50 tons of fuel. The oxygen itself will account for 78 per cent propellant mass in a methane-oxygen fuel mixture. Instead of lugging this excess baggage in the onward trip, liquid oxygen for the fuel could be prepared on Mars.
Science fiction writers imagine that in future, as a first step, a small nuclear reactor along with a scaled-up version of the MOXIE instrument would be sent to the Red Planet. Working 24.5×7 (the ‘day’ on Mars called ‘sol’ is a bit longer than the length of the day on Earth), the oxygen tank is filled up in preparation for the human visitors. When the explorers arrive, the power source, fuel for the return journey and life support infrastructure are already in place.
While human travel is far away, in the short run, the technology could be a boon for robotic return missions, say bringing back samples from Mars. MOXIE is also a small step towards the ‘In-Situ Resource Utilization (ISRU)’ plan.
With new technologies like 3D printing and additive manufacturing, in-situ resource utilisation can be expanded to include not just oxygen but also other materials. Instead of taking all the requirements, through the ISRU, one can satisfy a significant part of the materials needs from in-situ resources.
Cleaning up Earth’s atmosphere
Close to home, the same technology holds potential to mitigate global warming. If we can pluck carbon dioxide from the Martian air, why not scrub it off Earth’s atmosphere?
It is outlandish to believe the SOEC technology as a magic wand that will make the carbon in our atmosphere disappear. We need firm action on a global scale, like the elimination of the Ozone-depleting substances under the Montreal protocol. Nothing less will suffice. However, technologies can play a modest role in the mitigation of global warming. There are several ways the technology can be tweaked to provide moderate relief.
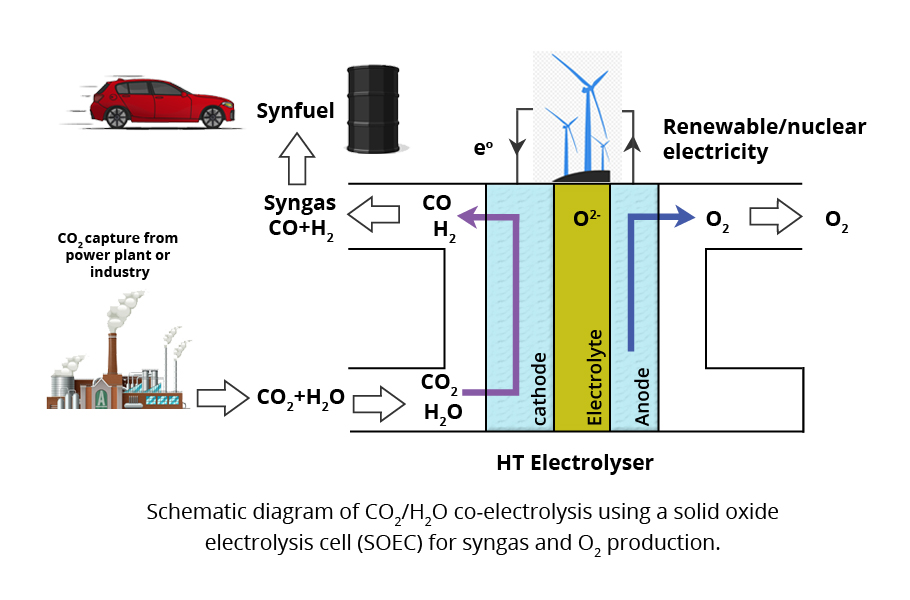
Non-renewable are notoriously uneven. The wind is copious during certain seasons when the production of the electricity far exceeds the demand, but is next to nothing during certain months. One can harness the excess power for electrolysis of water. One of the products of the electrolysis—hydrogen—can be stored, using a fuel cell and reconverted again when the demand arises.
If water and carbon dioxide are electrolysed at the same time, we get a mixture of hydrogen and carbon monoxide at cathode and oxygen at the anode. The combination of hydrogen and carbon monoxide, called syngas, can be used to syntheses of hydrocarbons. An alternative to the current petroleum-based liquid fuels can be made from syngas, reducing the carbon footprint. The carbon emitted by the syngas fuel would be the feedstock for the production of the syngas.
Thus, the net emitted carbon into the atmosphere will be zero. But the catch is the energy input needed for the electrolysis. One can use the wind turbines, solar cells, or any of the renewable sources to drive the electrolysis. Alternatively, syngas can be co-produced in industries that emit carbon by harnessing the waste heat of the industry.
But for all that to happen, there is still a long time to go.

