
- Home
- India
- World
- Premium
- THE FEDERAL SPECIAL
- Analysis
- States
- Perspective
- Videos
- Sports
- Education
- Entertainment
- Elections
- Features
- Health
- Business
- Series
- In memoriam: Sheikh Mujibur Rahman
- Bishnoi's Men
- NEET TANGLE
- Economy Series
- Earth Day
- Kashmir’s Frozen Turbulence
- India@75
- The legend of Ramjanmabhoomi
- Liberalisation@30
- How to tame a dragon
- Celebrating biodiversity
- Farm Matters
- 50 days of solitude
- Bringing Migrants Home
- Budget 2020
- Jharkhand Votes
- The Federal Investigates
- The Federal Impact
- Vanishing Sand
- Gandhi @ 150
- Andhra Today
- Field report
- Operation Gulmarg
- Pandemic @1 Mn in India
- The Federal Year-End
- The Zero Year
- Science
- Brand studio
- Newsletter
- Elections 2024
- Events
- Home
- IndiaIndia
- World
- Analysis
- StatesStates
- PerspectivePerspective
- VideosVideos
- Sports
- Education
- Entertainment
- ElectionsElections
- Features
- Health
- BusinessBusiness
- Premium
- Loading...
Premium - Events
How single-celled creatures became multicellular
While these studies have established cell colonies can emerge in certain conditions, it is a long road towards the evolution of true multicellular

Until about two billion years ago, since the origin of life, the Earth was teeming with just single-celled creatures, all of whom were living the life of a hermit, each tending their business of finding food and reproducing, unmindful of their fellow beings. One day, revolution happened. A chance mutation made daughter cells not separate from the mother cell even after cell division was...
Until about two billion years ago, since the origin of life, the Earth was teeming with just single-celled creatures, all of whom were living the life of a hermit, each tending their business of finding food and reproducing, unmindful of their fellow beings.
One day, revolution happened. A chance mutation made daughter cells not separate from the mother cell even after cell division was complete. This small change led to a giant leap. Giving up individual independence, some single-celled creatures joined hands to become a multicellular organism for the common good.
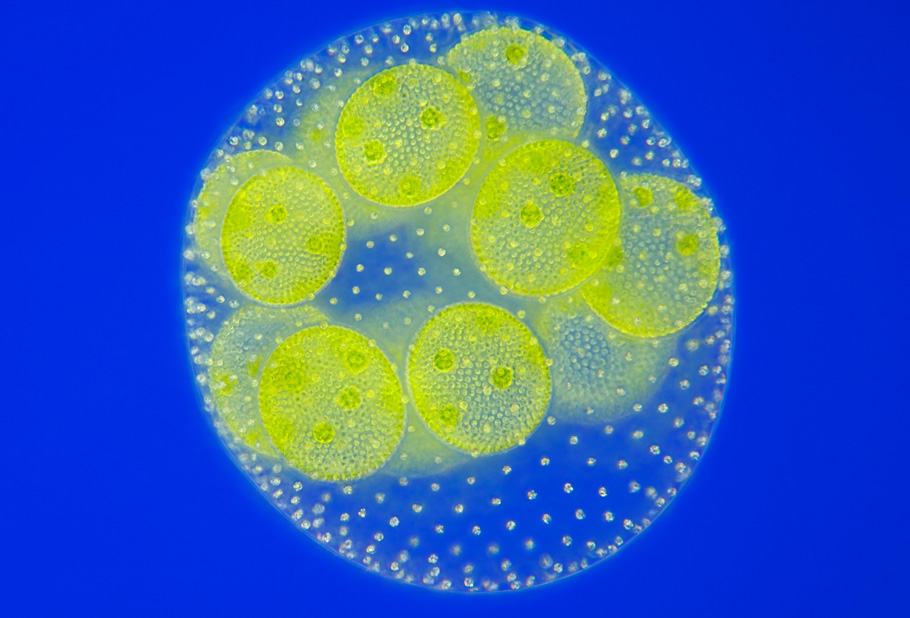
Coenobia of the chlorococcal alga has eight cells; Humans have a whopping 37 trillion. Typically, animals have a greater diversity of specialist cell types, around 100–150 (200 in humans), compared with 10–20 in plants. The evolution of multicellularity has brought in so much diversity in life.
The first known single-celled organisms appeared on Earth about 3.5 billion years ago, roughly a billion years after Earth formed. Although a 3 billion-year-old fossil record of the mats of ancient microbes, a 2-billion-year-old, coil-shaped Grypania spiralis fossils, hint at appearance of multicellularity, they were most likely cell clusters.
Yet, it took a long time for the true multicellular to emerge on the scene. An actual multicellular organism acts as a unit. Meaning cells in the cluster lose their individuality and work to benefit the whole. Evidence for this does not appear until about 600 million years ago. Fossiles of Sponges (750 million years ago), the first large and complex multicellular soft-bodied Ediacaran fossils (570 million-year-old), are the earliest. Multicellular plants perhaps appeared around 470 million years ago.
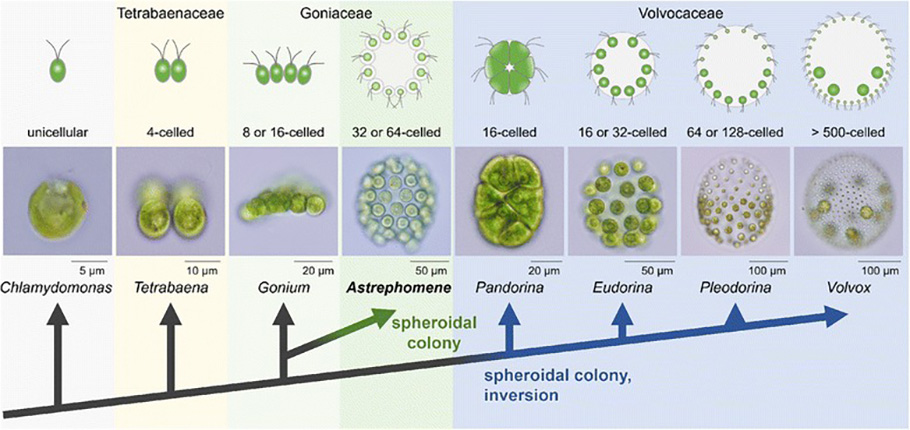
In the history of the evolution of life, the cellular aggregation has occurred at least 25 times. More robust multicellularity with sustained cell to cell interconnection, communication cooperation has evolved multiple times in bacteria, three times in Fungi, six times in algae and only once in Animalia.
But scientists have long been puzzled about what triggered this transition. In recent years, scientists, like Professor Lutz Becks at the Limnological Institute of the University of Konstanz, Germany, are gaining insights by recreating the evolution of multicellularity in the lab.
A team led by him cultured a unicellular green alga, Chlamydomonas reinhardtii. Over only 500 generations to establish the emergence of the first steps towards multicellularity. They experimentally demonstrated that when the cell clusters are better at reproduction and more likely to survive than single cells, then the germination of cell clusters leads to multicellularity.
If it ain’t broke, don’t fix it
All by themselves, the single-celled creatures lived a carefree life for millions of years. They roamed in any direction they wished, and if luck would have it, they found food. If they got adequate nourishment, they matured and reproduced. Except when falling prey to a predator or starved to death, these unicellular creatures were immortal by perpetually giving birth to daughter cells.
Once they entered wedlock and commenced living a communal life with other cells, the individual had to partially give up freedom to remain bonded with others. In an advanced multicellular organism, the cells underwent ‘differentiation’ and specialised in a particular function. In these multicellulars, only the germ cells reproduced. Most of the other cells, called soma cells, give up their own reproduction for the greater good, thriving only to fulfil their duty to the collective. When these soma cells rebelled, cancer erupted.
Cooperation is not all that unpleasant. Friends came with benefits. Cell conglomerates could develop specialised multicellular organs like jaws, claws, tentacles, eyes, roots, leaves or fingers. Unlike a typical single cell that tumbled in a limited environment, a multicellular unit could travel great distances to seek nutrients or more favourable ecological space.
Definitely, after the emergence of multicellular, it may be advantageous to the species, but how did the unicellular entities develop into a multicellular unit in the first place? The gulf between the single-celled and multicellular seems too wide to bridge.

Piecing the puzzle of the evolution of life is greatly aided by the rich fossil records. However, as the early forms of multicellular organisms lacked hard body parts, there are hardly any fossil records to go by. But surprisingly, recent novel studies using an approach known as experimental evolution drive microorganisms to aggregate into clusters, the first step in the evolution of multicellularity.
Till death do us part
In hindsight, the development of multicellularity appears as a giant leap in evolution. Still, according to the standard multilevel selection (MLS) model, each transition involved a series of small advances. First, the single-celled creatures had to evolve effective ways to stick together. Then the cells in the conglomerate had to cooperate and not drag the colony in a different direction. Finally, each cell had to develop a specialised function within the greater whole and work towards the greater good.
In 2014, William Ratcliff, a biologist at the Georgia Institute of Technology in Atlanta, and his collaborators demonstrated that in just 100 generations, Saccharomyces cerevisiae, yeast could be goaded to become a colonial.
The researchers grew the microbes in tubes and spun them in a centrifuge once a day. While some yeast cells floated on the top, others sunk to the bottom. They filtered the fast sinkers from each of the test tubes and cultured them afresh in test tubes. Once again, after 24 hours, they spun these test tubes, harvested fast sinkers for further cultivation. They repeated this for 60 days.
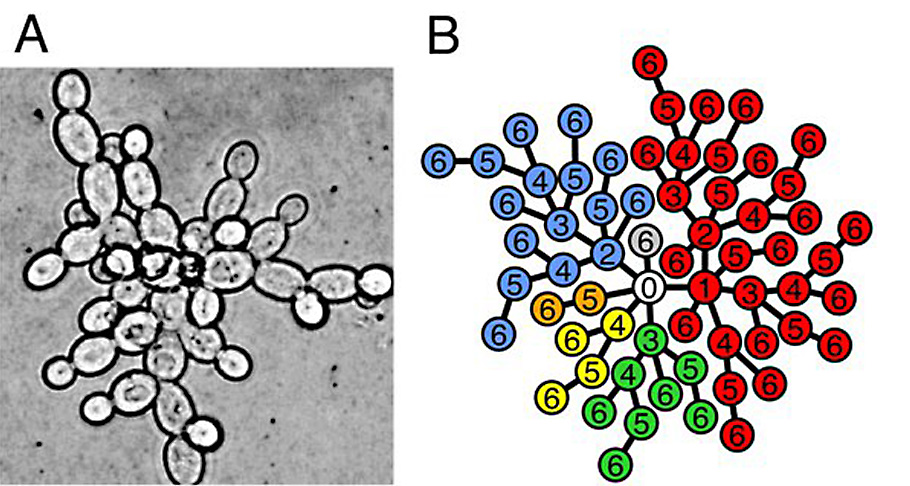
The single-celled organisms, like yeast, multiply by dividing. In this process known as binary fission, a single cell splits into two daughter cells once they mature. One cell divides into 2, 2 cells into 4, 4 into 8, 16, 32 64 and so on. As the cells split, the DNA copies are made, random errors, mutations, takes place. In 24h, the yeast undergoes almost seven life cycles, and tens of thousands of mutation takes place during this time in the test tube. Most of the mutations are silent and have no effect. Some are deleterious, and the mutation along with the cell perish. Some give the cells specific new characteristics.
One striking mutation the researchers observed was daughter cells cling to the mother cells. While typical yeast divides and disperses after each generation, these mutants cells divide and stick. As a rare occurrence, some twins, about one in 49,000 births to 1 in 189,000 births, are born as conjoined twins, with both babies are born physically connected to each other. Likewise, at times the daughter cells remained conjoined. Daughter cells protruding from the mother cell gave the cluster an appearance of a snowflake.
Clusters of cells sticking with each other settle more rapidly than single cells; they had a better chance of selection in each cycle. As little as 100 yeast generations, the population in some of the test tubes had shifted almost entirely to snowflake yeast in two weeks.
Although the clusters survived the predator, perhaps it was at the cost of their own reproduction success. The cell clusters settle faster and have a reproductive advantage over single cells. While the cells at the periphery would have unhindered access, the interior cells are handicapped in accessing food. Thus, as a whole, these two tendencies may drive reproductive success to low levels as a trade-off between reproduction and survival.
While Ratcliff’s experiment established clusters could naturally emerge, that was only the first step towards multicellularity. One needed to show that the clustering is a heritable characteristic. Further, the cell groups must be more salutary at reproduction and more fit to survive than single cells.
Recently Professor Lutz Becks at the Limnological Institute of the University of Konstanz, and his colleagues, in collaboration with a researcher from the Alfred Wegner Institute (AWI), embarked upon exploring this.
Cooperate to dominate
Like Redcliff’s experiment here, too, the alga Chlamydomonas reinhardtii was grown in the laboratory in test tubes. Not one but ten different cell lines of the alga were isolated and cultured. Some of the test tubes introduced a multicellular rotifer, a microscopic aquatic animal, a natural predator of the alga. Control tests tubes were free of rotifer or any adversary.
They run the experiment for six months, and by then, algae which have a lifetime of nine hours, underwent 500 generations. They compared the test tubes and classified the cells into six different types. They observed that some of the cells, growing in the presence or absence of the predator, lost their flagella. This structure aids the microorganism like an oar to swim towards the food and away from the danger. While others retained motility. As anticipated, after many generations, in this experiment too, chance mutation led to variants in which daughters stick together after cell division and form cell clusters.

A chance observation gave a hint for the researchers to come up with this experimental design. “We initially observed cell groups in an experiment with rotifers and the algae where we did not expect them and didn’t know how to deal with them, as the colonies interfered with getting cell counts. We quickly realised that growth in cell groups can be advantages and that it is heritable,” says Lutz Becks.
Hunted by the predator, rotifer, the chance mutation gave the cluster algae a better chance of survival. “The mouth opening of the rotifer is approx. 30 micrometer and the single celled algae has a diameter of ~ 5 micrometer,” says Becks. Naturally, the loner algae were easy prey. At the same time, the probability of survival of colonies increase because predators can no longer, or at least not as quickly, eat the colonies. In about 6 hours, eight daughter cells clustered, making o big that they became too large for predators. In these test tubes, loners or few algae cells huddling together had little chance to escape the assailant.
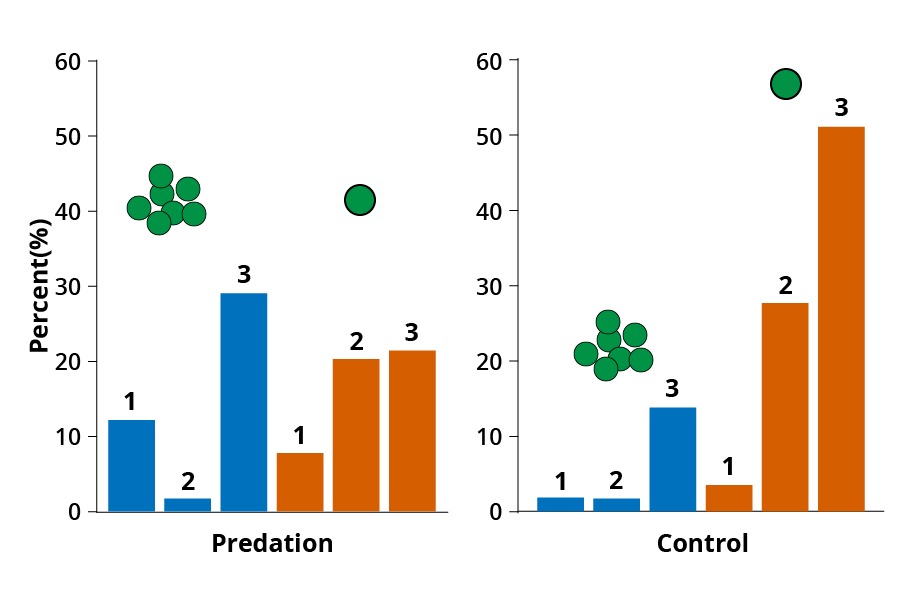
While there were a smattering of cell clusters even in test tubes without the predator, after 500 generations, cell cluster was dominant in test tubes with predators. In test tubes, clustering does not give any advantage, only a handicap. But in test tubes with predators, it is a life or death question. The trade-off between high survivability and low reproduction could be clearly seen by comparing the test tubes with and without predators. This showed that colonies grew significantly more often in the presence of predators and had a considerably higher reproductive rate than colonies growing without predators.
With passing generations, the test tubes with predator exhibited cells groups. However, rotifers starved; their numbers dwindled, indicating successful survival by the cluster cells. Lower rotifer growth rate = higher algal survival. Concomitantly, cells in the inner part of the clusters had less access to food and, hence, lower resource uptake. Further, as the daughter cell does not separate after cell division, usually they lose motility. All these factors combined with decreasing the lower reproduction rate among the clusters.
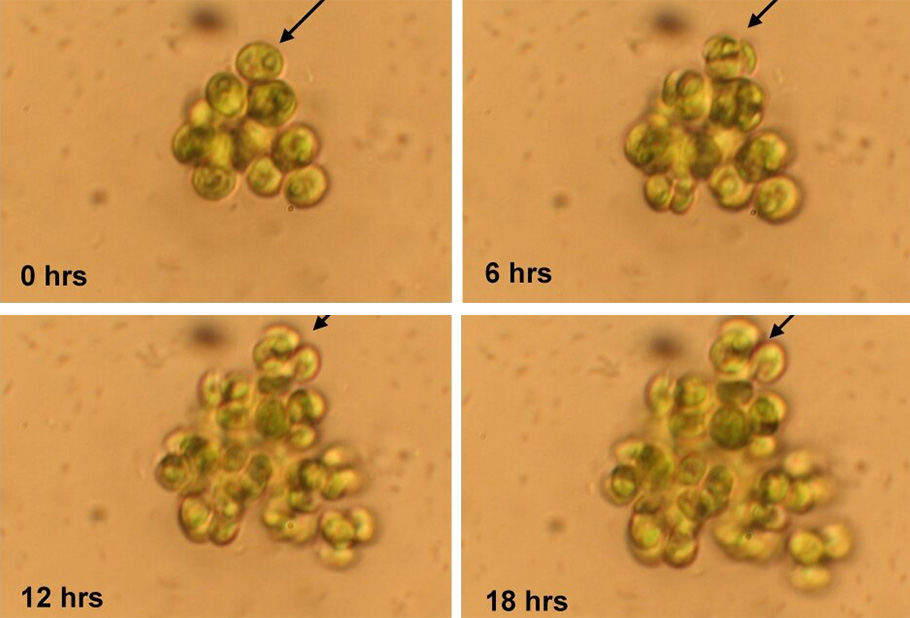
How are we sure that the cell clusters are heritable. After twenty generations, they took groups and single cells and grew them in a shared environment. If the clustering was only a short term tactic and not a heritable feature, one would expect the clusters to dwindle without a specific advantage. But that did not happen; the colonies gave birth to colonies.
They also conducted gene sequencing to identify potential candidate genes involved in cell groups. One genetic mutation may be driving cell clusters in one test tube. At the same time, it is a very different genetic mutation that prompts clustering in another test tube. To investigate, the researchers sequenced whole genomes of nine pairs of clones from predation and no-predation selection experiments.
The genomic study surprised everyone. “We had actually expected that the formation of colonies can be achieved by different mechanisms in the algal cells and we would therefore find different mutations. In fact, we have seen a very high level of repeatability. This suggests that the selection pressure has had a very targeted effect,” says Lutz Becks in a press release.
Genome sequencing established many genes were expressed differentially between cell groups and single-cell clones. Moreover, genetic changes were seen in genes involved in cell adhesion, nutrient uptake, and stress response to CO2 in the cell groups. All these changes provide a specific advantage to the cells living in clusters.
A journey of a thousand miles begins with a single step.
One of the significant enigmas of evolutionary theory is how complexity and organisation emerge without an intelligent design. These lab-simulated evolution studies suggest that, like a blind watchmaker, random mutations and natural selection can trigger complexity. These novel experiments demonstrate that even without conscious goal or direction or systemic intentionality, evolutionary trajectory emerges.
These lab studies use evolved unicellular, and specifically, in Lutz Becks’s study, a predator is a multicellular organism. How far these actually mimic the early Earth is an important question. “There are unicellular predators (flagellates, ciliates) that feed on unicellular algae. There is also experimental work, that shows the formation of colonies in response to predation by unicellular predators,” explains Lutz Becks.
“Note that cell groups as we found in our study are not multicellular organisms, but one of the steps”, cautions Lutz Becks. While these studies have established colonies can emerge in certain conditions, it is a long road towards the evolution of true multicellular. Further, he says that “any selection pressure that provides a benefit to the larger cell groups could in theory select for cell groups”, driving towards the emergence of true multicellular organisms.
Nevertheless, more clarity on how these clusters become more complex, break down tasks and assign them to different cells like a well-run assembly line is yet to emerge. For example, the taillike flagella used by the single-cell Chlamydomonas to move is present in each of the daughter cells in a cluster. However, many of these are trapped inside the cluster and useless for the cell or group. The algae clusters have to evolve ways to get the flagella of peripheral cells at the right place and coordinate their movement if they have to go somewhere.
