Expert panel to review Bharat Biotech's ‘Covaxin’ for emergency use nod
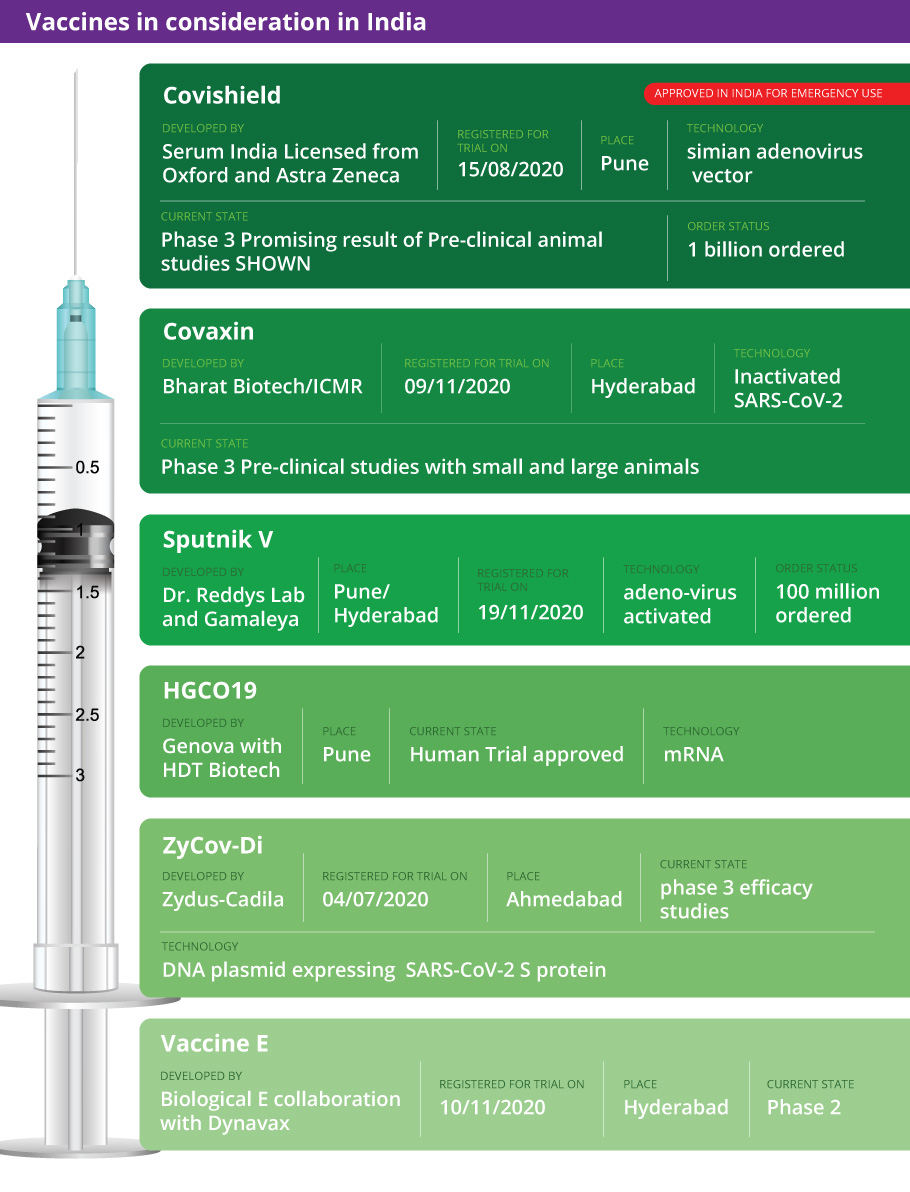
A day after the Subject Expert Committee cleared Oxford-AstraZeneca-Serum’s ‘Covishield’ and sent it to the Drugs Controller General of India (DCGI) for final approval, Bharat Biotech's ‘Covaxin’ will be reviewed for emergency use on Saturday (January 2). Notably, Covaxin is India's only COVID-19 shot developed indigenously in association with top medical research body Indian Council of Medical Research (ICMR).

A day after the Subject Expert Committee cleared Oxford-AstraZeneca’s ‘Covishield’ and sent it to the Drugs Controller General of India (DCGI) for final approval, Bharat Biotech’s ‘Covaxin’ will be reviewed for emergency use on Saturday (January 2). Notably, Covaxin is India’s only COVID-19 shot developed indigenously in association with top medical research body Indian Council of Medical Research (ICMR).
Besides ‘Covishield’ and ‘Covaxin’, US pharma major Pfizer, too, has applied for emergency use in India. The company sought more time to present its data before the panel of experts. The Pfizer vaccine has been approved by the World Health Organization.
Also read: Oxford’s Covishield gets expert panel nod, final call with regulator
‘Covaxin’, though made in India, is still lagging behind others as it has failed to get enough volunteers for its final trials. Bharat Biotech was supposed to complete its trials by December 31, but has extended the date for registration. Till December 22, Bharat Biotech was able to recruit only 13,000 out of the required 26,000 candidates for the final-stage trials.
Covaxin may not get a go ahead since its phase III data, which proves efficacy of the vaccine, is not available so far. Encouraging news is that phase 1 and 2 data shows that the vaccine is safe with no side effects, experts said.
A dry run of the inoculation process is happening across the country to check on-field preparedness and feasibility of the CoWIN app — a digital platform to roll out and scale up the vaccination drive.