Oxford's Covishield gets expert panel nod, final call with regulator
Covishield vaccine, a joint venture of Oxford University-AstraZeneca-Serum Institute, is likely to get approval for emergency use in India, Reuters reported on Friday (January 1).

Covishield, the COVID-19 vaccine candidate developed by Oxford University and AstraZeneca, was recommended for emergency use approval to the drug regulator by the government-appointed panel on Friday (January 1).
The Central Drugs Standard Control Organization (CDSCO), whose experts met on Friday for the second time this week, have sent the vaccine for approval to the Drugs Controller General of India (DCGI).
Reports earlier in the day had speculated that the committee may also approve Bharat Biotech’s vaccine candidate Covaxin for emergency use. However, the committee’s recommendation for it is awaited.
Drugs Controller General Dr VG Somani had said on Thursday: “Probably, we will have a happy New Year with something in hand. That is what I can hint at”.
Also read: WHO clears Pfizer-BioNTech coronavirus vaccine for emergency use
Britain and Argentina have already authorised Covishield for emergency use.
The government has asked all states to conduct a dry run of vaccination on Saturday (January 2), indicating that it is preparing for a nationwide rollout of a vaccine soon.
India is the second worst affected country in the world by the COVID pandemic after the United States. India intends to vaccinate 30 crore people in the coming 6-8 months and the Oxford vaccine, which is the most affordable, is the best option at hand.
Also read: Nod for COVID vaccine soon? Centre tells all states to conduct dry run
Serum Institute of India (SII) has already stocked more than 5 crore doses of the AstraZeneca vaccine and the jabs could be transported to all states starting Saturday, Reuters reported.
Pfizer Inc asked for more time to showcase its data for emergency authorisation of a vaccine it has manufactured in association with Germany’s BioNTech.
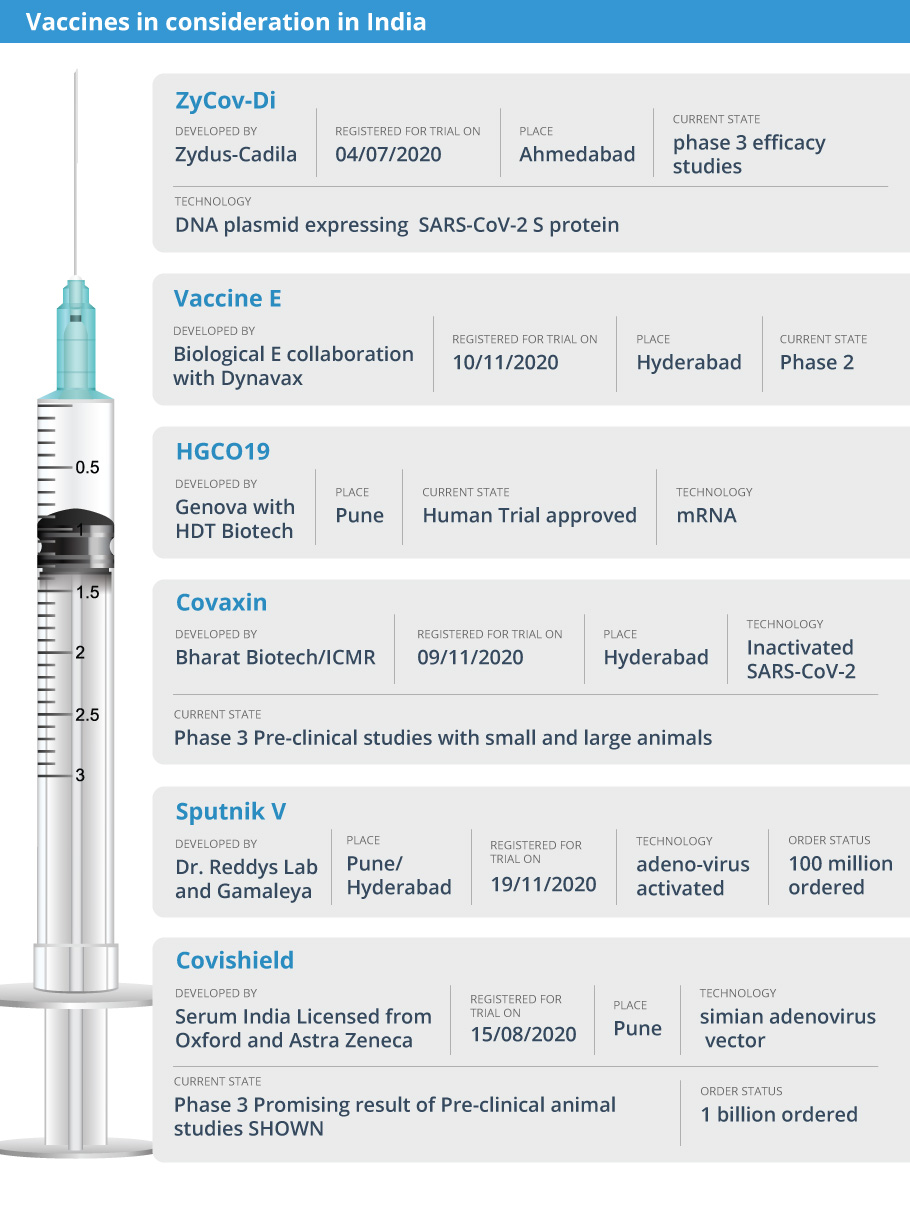
Here are the vaccines for COVID in different stages of development in India: