
- Home
- News
- Analysis
- States
- Perspective
- Videos
- Education
- Entertainment
- Elections
- World Cup 2023
- Features
- Health
- Budget 2024-25
- Business
- Series
- NEET TANGLE
- Economy Series
- Earth Day
- Kashmir’s Frozen Turbulence
- India@75
- The legend of Ramjanmabhoomi
- Liberalisation@30
- How to tame a dragon
- Celebrating biodiversity
- Farm Matters
- 50 days of solitude
- Bringing Migrants Home
- Budget 2020
- Jharkhand Votes
- The Federal Investigates
- The Federal Impact
- Vanishing Sand
- Gandhi @ 150
- Andhra Today
- Field report
- Operation Gulmarg
- Pandemic @1 Mn in India
- The Federal Year-End
- The Zero Year
- Premium
- Science
- Brand studio
- Home
- NewsNews
- Analysis
- StatesStates
- PerspectivePerspective
- VideosVideos
- Entertainment
- ElectionsElections
- Sports
- Loading...
Sports - Features
- Budget 2024-25
- BusinessBusiness
- Premium
- Loading...
Premium

For COVID-19 cure, WHO unites nations for global study
As the coronavirus is new, it is not surprising that there are no drugs against it. At present, the treatment for COVID-19 is primarily supportive care, including ventilation if necessary.

In a move to accelerate clinical research to find a better treatment for COVID19, the UN World Health Organization (WHO) has launched an unparalleled global study, ‘SOLIDARITY trials’. India will be part of this global effort to simultaneously test four potential therapies by comparing the efficacy of each of them. Lauding it as an “unprecedented opportunity to come together as one...
In a move to accelerate clinical research to find a better treatment for COVID19, the UN World Health Organization (WHO) has launched an unparalleled global study, ‘SOLIDARITY trials’.
India will be part of this global effort to simultaneously test four potential therapies by comparing the efficacy of each of them.
Lauding it as an “unprecedented opportunity to come together as one against a common enemy”, Tedros Adhanom Ghebreyesus, Director-General of WHO, said, “This large, international study is designed to generate the robust data we need to show which treatments are the most effective.”
The guidelines issued by the Indian health ministry candidly admits that “no specific antivirals have been proven to be effective as per currently available data”. In the absence of reliable medication, supportive care remains the only treatment provided to very ill COVID 19 patients.
With the viable vaccine at least 12–18 months away, to save the lives of severely ill patients, there is an urgent need to find effective therapeutics. The strategy of ‘SOLIDARITY trials’ is designed to speed up a process by examining four different therapies that involve repurposing the existing drugs for other viral diseases.
In addition to India, countries across the world such as Norway, Spain, China, Argentina, Bahrain, Canada, France, Iran, South Africa, Nepal, Thailand will participate in the “Solidarity trial”. One notable but predicted exception is the United States of America.
COVID-19
COVID-19 is a condition caused by the coronavirus (called SARS-CoV-2) that was first identified in Wuhan, China in late 2019. The virus infects the respiratory system, particularly the lungs. A large proportion of the infected people do not show any symptoms but spread the germ to others.
Many develop mild diseases which include fever and cough. A few may develop pneumonia. Due to the chest infection, the small air pockets of the lungs, called alveoli, are filled with liquid making it difficult to breathe. If the disease worsens unabated, then lung tissues are damaged, leading to respiratory failure or respiratory failure combined with heart damage.
As the virus is new, it is not surprising that there are no drugs against it. At present, the treatment for COVID-19 is primarily supportive care, including ventilation if necessary.
Coming up with a new drug molecule from ground up will take years. Given the urgency, researchers are dusting up existing antiviral drugs to treat the critically ill. These include medications used for treating HIV virus and molecules found to be effective in animal studies against the related coronavirus diseases.
Medicines used for treating severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) show promise. A drug developed to arrest the Ebola outbreak offers possibilities. All the medications available are pulled out from dusty cabinets to provide an effective treatment to the COVID19.
Prevent the freeloaders
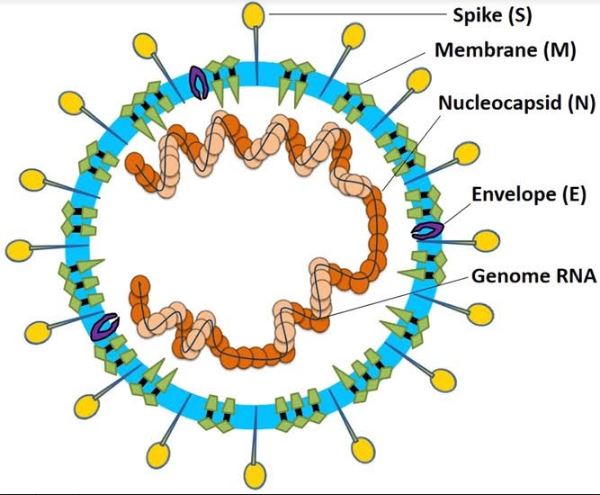
Novel coronavirus (SARS-CoV-2) is like a freeloader with a big appetite. SARS-CoV-2 contains just a strand of RNA (a molecule that stores genetic information) and four different proteins. The genetic code in the RNA has the step by step instruction for preparing the proteins it needs and also the instructions for making a copy of itself.
Like a theoretician lacking practical tools, the virus does not have the tools and machinery, kitchen or ingredients, to make its meal. It has to raid a restaurant, a human cell, gain entry deceptively, place an order for the dishes it wants, replicate, and exit the restaurant before being caught.
Most viruses depend on the acidification of endosomes, a cell organelles (or part), for gaining entry into the cell. Like a bouncer blocks the entrance, antimalarial drugs chloroquine and hydroxychloroquine thwart the entry of the virus by decreasing the acidity of the endosomes.
Although extolled by US President Donald Trump as a ‘game-changer’ and many countries rushing to include it in the bouquet of therapy, the results from the cell culture studies are not that promising.
One, the dosage needed to arrest the entry of SARS-CoV-2 virus is so high that it could cause acute toxicities. Second SARS-CoV-2 virus gains entry by latching on to ACE2 receptor and binding with the tmprss2, a protease.
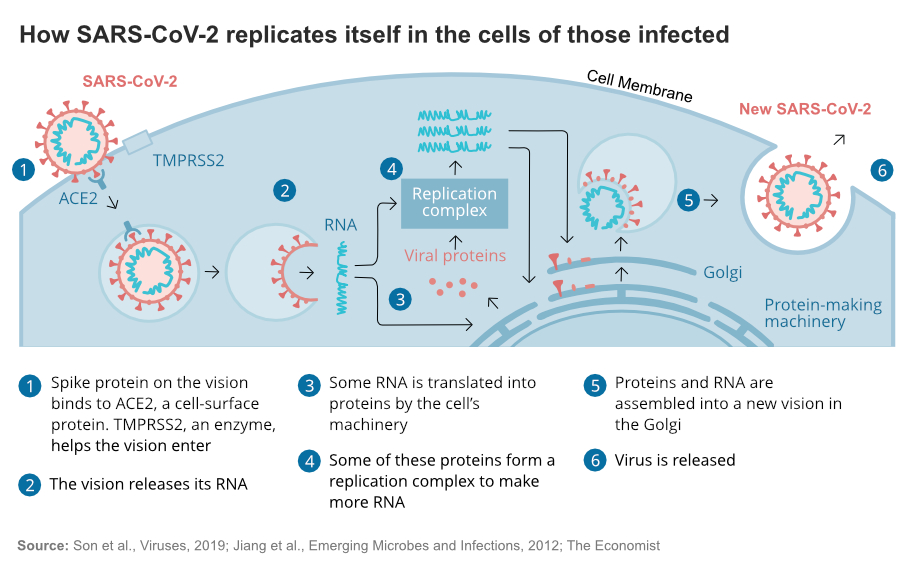
During the COVID19 outbreak, the Chinese experimented with chloroquine, and the French did a study which showed that the patients administered with hydroxychloroquine had significantly reduced viral load in the nasal swabs. As nighter of these studies is randomised control clinical trials, researchers under the WHO have taken up these drugs for a rigorous clinical trial.
Mess up the cooking
As a virus is incapable of cooking the four proteins by itself, it presents the instruction for making the proteins to the cellular chef, the ribosome. Unaware that the virus is a freeloader, the ribosome diligently prepares the required proteins in a chain called polypeptide chains.
Just as a baked cake has to be sliced into chewable pieces, often the polypeptide chains have to be cut at the right places to get the required proteins. Snap at the wrong place, it is no longer a useful protein but a junk. Called proteolysis, if this cellular process is disturbed, the preparation of the dish is foiled. In the absence of the right proteins, the virus will not be able to replicate.
The combination drug ritonavir/lopinavir, second in WHO study, inhibits the protease. The combination drug has the approval of USFDA for treatment of HIV infection and is found safe for use in humans. Lopinavir disrupts the proteolysis; however, it is easily broken down in the human body. To make it stay longer, lopinavir needs to be fortified along with a low dosage of ritonavir.
Although routinely used in HIV patients, clinical trial among the SARS and MERS patients are ambiguous. The combination drug was used in Wuhan, China, for COVID-19 patients. Initial results are not that encouraging. However, it was administered to patients who were very ill. The SOLIDARITY study plans to study the efficacy of this drug at an earlier stage of the COVID 19 disease.
Neuter the parasite
Viral proteins are just a side dish. The main course is the viral RNA. A chef might be talented in multi-cuisine, Chinese, Indian or Mughlai, but will be clueless in preparing a cattle feed or fish meal. The ribosome is capable of reading an RNA and making the proteins. Day in day out that is what they do. During mitosis, they also copy the DNA. However, the cell never needs to make a copy of an RNA. What researchers could try here is deliberately bungling the synthesis of a complementary RNA molecule using an RNA template.
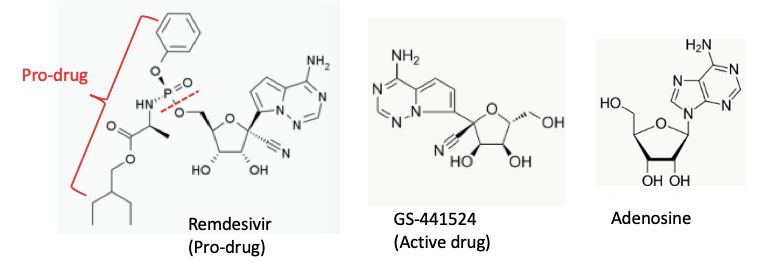
Remdesivir works by blocking the RNA polymerase that coronaviruses and related RNA viruses need to replicate their genomes and proliferate in the host body. It was initially developed during the Ebola outbreak but did not prove useful in clinical trial against Ebola virus.
Remdesivir, in technical terms, is a nucleotide analogue, meaning it looks like a key ingredient, but with a significant deformity. The south Indian vada and doughnut may look alike but are chalk and cheese. The active drug called GS-441524, a part of the inactive pro-drug Remdesvir resembles adenosine. When the cellular machinery stars using this ‘wrong’ component to build a copy of the viral RNA, the cookie crumbles. The nascent viral RNA, with faulty component prematurely terminates. Replication of viral RNA copies cease.
Although it did not prove useful in clinical trial against Ebola virus, Remdesivir a compound that shuts down viral replication by inhibiting an essential viral enzyme, the RNA-dependent RNA polymerase, will be tested with COVID-19 patients.
Alerting neighbours
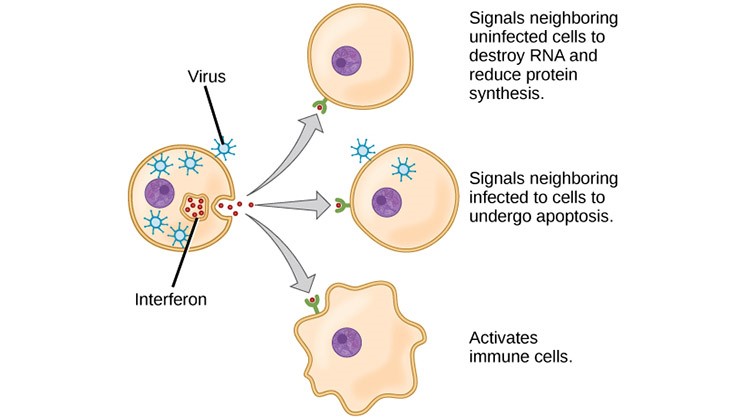
The sounding alarm of a burglary helps, your neighbours are forewarned. Interferons, a class of ‘signalling’ proteins produced and released by cells in response to infections do precisely that.
Once infected by a virus, the cell releases the distress signal. Interferon prompts the nearby infected cells to self-destruct and prevent the virus multiplying and invading many more cells. Uninfected cells are alerted to heighten their defences against the infection.
First identified by Jean Lindenmann and Aleck Isaacs in 1957, Cuba pioneered in industrial-scale production using recombinant biotechnology. The SOLIDARITY trials will also test the combination of interferon-beta along with the ritonavir/lopinavir.
Researchers hope to arrest the infection by combining the ritonavir/lopinavir’s inhibition of viral reproduction and interferon triggered heighten the antiviral activity. However, the use of interferon-beta on patients with severe COVID-19 might be risky. In a critical patient, the body has unleashed a cytokine storm.
In this all-out war against the invading virus, uninfected body cells are destroyed along with the infected cells. Administering interferons at this late stage would only worsen the tissue damage. The trials would examine applying the combination at an early stage to prevent the infection from spreading deep.
Global trial
Each of these four drugs is vouched by one or other medical practitioners, but the evidence so far is anecdotal. Evidence-based scientific medicine demands at least randomised trial before a drug is accepted genuinely having therapeutic value.
Multiple small trials, each conducted with different methodologies will not only take time but also may not provide the evidence needed. For a transparent verdict, clinical trials have to be undertaken on several thousands of patients. By going global, recruiting patients for trial from different countries, the robust clinical trial can be conducted to identify effective therapy.
After informed consent is obtained from the COVID 19 patients, each hospital will send the data to the WHO. By random allocation, each of the patients will be advised to be provided by one of the following five clinical treatment; 1. Local standard of care alone or local standard of care plus one of 2. Remdesivir 3. Chloroquine or hydroxychloroquine 4. Lopinavir + ritonavir 5. Lopinavir + ritonavir plus interferon-beta. The effect of the treatment on the number of deaths, duration of hospital stay and the time to first receiving ventilation would be measured.
The design is not double-blind, the gold standard in medical research. But WHO says it had to balance scientific rigour against speed. Although the placebo effect cannot be ruled out in this study, the randomisation will ensure removal of the trial allocation biases. It will be essential to get answers quickly, to try to find out what works and what does not. One or more of the drugs may reduce the severity of COVID-19, lessen the need for ventilation, and reduce the risk of death.
With grim statistics of galloping numbers of infected, escalating deaths, the clock ticking. The SOLIDARITY trials offer a silver ray of hope.

