Stop advertising, give research details: AYUSH ministry orders Patanjali
The Ministry of AYUSH on Tuesday (June 23) ordered Ramdev Baba's Patanjali to stop advertising it's COVID drug until "issue" is examined.

The Ministry of AYUSH on Tuesday (June 23) ordered Ramdev Baba’s Patanjali to stop advertising it’s COVID drug until the “issue” is examined.
Herbal medicine company Patanjali Ayurveda on Tuesday claimed to have discovered cure for coronavirus but no medical authority could immediately vouch for the claim of Coronil and Swasari medicine curing the highly contagious disease within seven days.
The firm claimed that the two Ayurveda-based medicines have shown 100 per cent favourable results during clinical trials on COVID-19 infected patients except those on a life support system.
AYUSH ministry says advertisement of Patanjali’s alleged drugs regulated under Drugs and Magic Remedies (Objectionable Advertisements) Act, 1954.
“Patanjali has been asked to provide at the earliest details of name and composition of medicine being claimed for COVID-19 treatment. We have also asked details of sample size, sites, hospitals where research study was conducted, and ethics panel clearance,” the ministry said.
अश्वगंधा, गिलोय और तुलसी की मात्रा बताई डॉक्टर ने उसी के एक्टिव कंपाउंड को लेकर हमने यह कोरोनिल नाम की आयुर्वेदिक औषधि इस संसार को कोरोना मुक्ति के लिए एक उपहार के रूप में दी है।
पूज्य @yogrishiramdev जी pic.twitter.com/y20RONfgfm— Patanjali Ayurved (@PypAyurved) June 23, 2020
The medicines have been developed by Patanjali Research Center, Haridwar and privately-owned National Institute of Medical Science, Jaipur following all protocols with clinically controlled trial-based evidence, Ramdev told PTI.
“Patanjali first conducted clinical case study and conducted clinical control trials following all protocols of drug discovery,” he said.
Sidestepping questions on the drug being approved by government agencies such as ICMR, Ramdev said clinical controlled study of these medicines was done in several cities including Delhi, Ahmedabad and Meerut and the RCT (Randomized Clinical Trial) controlled with placebo was conducted at Jaipur-based National Institute of Medical Sciences & Research.
“This was done after getting approval from Clinical Trial Registry of India (CTRI) and all other required formalities,” he said. “We have followed all the parameters set up by modern science for such clinical trials.”
Both the Ministry of Ayush and the Indian Council of Medical Research (ICMR) have reportedly refused to comment on the claim citing lack of knowledge of the issue.
A government notification bars companies from advertising a cure without government approval.
Ramdev claimed that his ayurvedic drugs have shown 100 per cent favourable results during the clinical trial.
At a launch press conference in Haridwar, he said, “The whole country and the world was waiting for the medicine or vaccine for coronavirus. We are proud to announce that the first ayurvedic, clinically controlled trial based evidence and research-based medicine has been prepared by the combined efforts of Patanjali Research Centre and National Institute of Medical Sciences (NIMS)”.
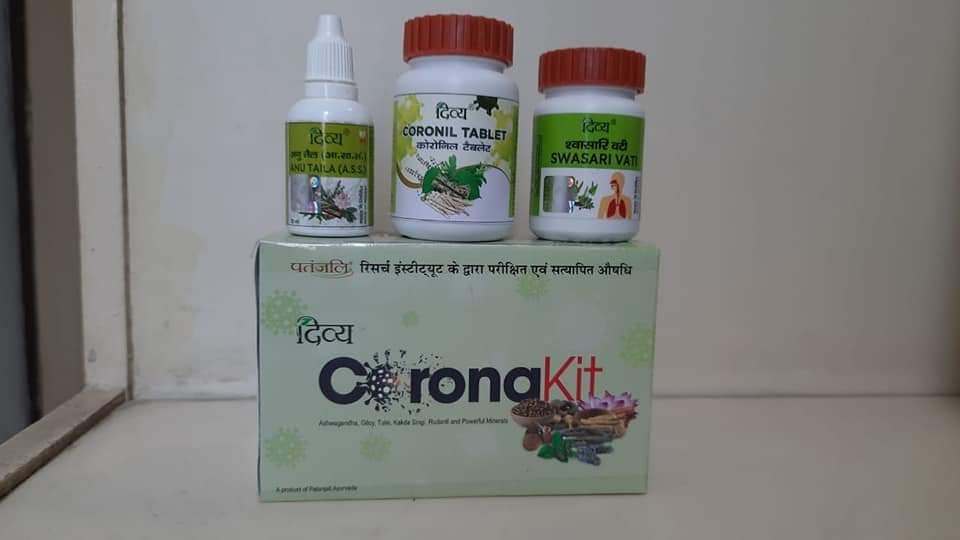
Related news: Patanjali launches ‘Corona kit’, claims 100% COVID-19 cure in 7 days
“We conducted a clinical case study and clinical controlled trial, and found 69 per cent patients recovered in 3 days and 100 patients recovered in 7 days,” he added.
According to him, this medicine is useful for prevention from COVID-19 and also for treatment. He claimed that no infected patient lost his life during the trial.
Ramdev also claimed that during the trial other complications of patients were also cured and their critical parameters were normalised.
“Even parameters as hsCRP and IL-6 levels were also controlled during the trials,” he added.
The test has been done on all kinds of patients except those who are highly infected and are on life support systems as ventilators.
“Trials on the infected people who are on a Ventilation support system are still due,” he said adding that the trial has been conducted only on a normal infected person and persons with medium infections.
Ramdev further claimed “severely infected patients, which are on a life support system, can also be cured by this medicine but trials for such cases are due in the second stage.” Patanjali had started to work on corona medicines in December 2019.
The Corona kit, priced at ₹545, would be available pan India within a week. The kit will have medicines for 30 days. This is not an immunity booster but a coronavirus cure, Ramdev said.
Manufactured by Haridwar-based Divya Pharmacy and Patanjali Ayurved Ltd, the medicine is the result of a research partnership between Patanjali Research Institute and the Jaipur based National Institute of Medical Sciences.
Ramdev said the medicines can be ordered online through a mobile app from next Monday.
The COVID-19 therapy suggested by Patanjali is applicable for adults ranging between 15-80 years of age whereas children are advised to take half the dosage prescribed for adults.
Patanjali’s anti-COVID tablet, Divya Coronil Tablet consists of Giloy, Tulsi and Ashwagandha and is prescribed to be taken thrice a day with hot water 30 minutes after breakfast, lunch and dinner.
Related news: Patanjali’s plea for Ayurvedic medicine trials on COVID-19 patients raises eyebrows
Medical experts, however, were not convinced by the claims made by Yoga guru Ramdev’s Patanjali.
Dr Ravi Shekhar Jha, Head of the department and senior consultant, Pulmonology, Fortis Escorts Hospital, Faridabad, said physiologically it is quite impossible that there is any medicine which can finish the viral load from the body in 5-7 days.
“These claims can be very harmful because people, instead of taking actual medical help, may start rushing to these kinds of claims which have no proven clinical trial,” he told PTI.
Instead of taking actual medical help, opting for a “desi nuskha” kind of a thing is just going to result in loss of precious time in curing the disease, Jha said.
Noted city-based lung surgeon Dr Arvind Kumar said only a proper clinical trial can prove the efficacy of the medicine.
Haridwar-based Patanjali group has an estimated turnover of around ₹10,500 crore. It had acquired debt-ridden Ruchi Soya in a corporate insolvency resolution process for around ₹4,350 crore after competing with Adani Group.
Patanjali Ayurved had reported a turnover of ₹8,329 crore in FY 2018-19.
However, the groups turnover was higher as Patanjali Ayurved consists of its FMCG business and ayurvedic medicines. Business like biscuits, noodles, dairy products, solar panel and its apparel segment Paridhan etc are not part of Patanjali Ayurved Ltd.
The group is also known for venturing in uncharted areas as it had in August 2018 launched a messaging app Kimbho, which could not took up well and is now defunct.
Related news: Can Siddha medicine be used in COVID-care? Docs say more testing needed
(With inputs from agencies)


