Hyderabad's Zenara Pharma to make & sell Paxlovid for mild Covid cases

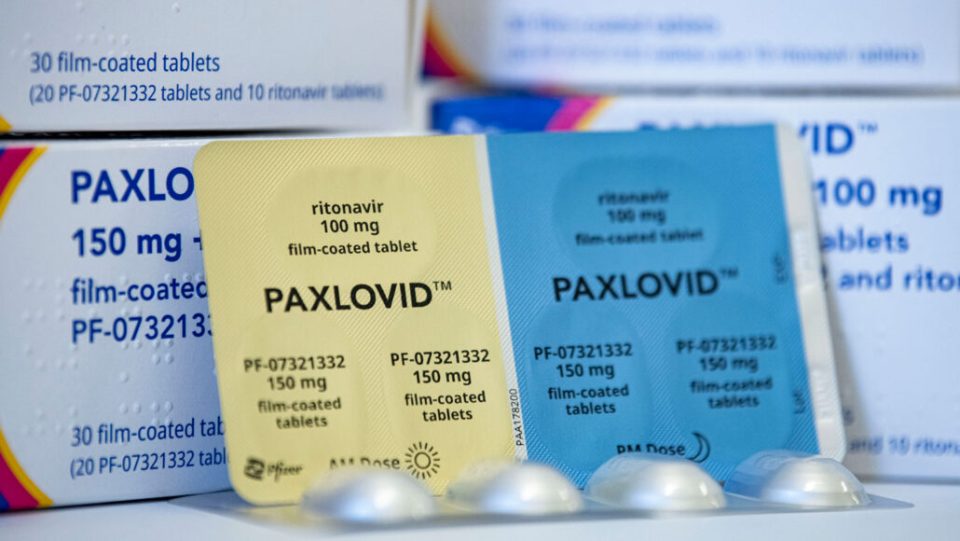
Drug maker Zenara Pharma has got approval to manufacture and market Paxlovid (Nirmatrelvir and Ritonavir) tablets in a combi pack as a treatment option for patients with mild to moderate symptoms of Covid-19, the company said in a press release on Friday.
The new product will be sold under the brand name ‘Paxzen,’ and is being manufactured at Zenara’s US FDA- and EU-approved state-of-the-art facility in Hyderabad.
Each box will contain 20 tablets of Nirmatrelvir 150 ml and 10 Tablets of Ritonavir 100 mg and each box will cost ₹5,200. Paxlovid (Nirmatrelvir and Ritonavir) tablets can be administered to patientens with mild to moderate symptoms of Covid-19.
Also read: COVID pills are round the corner; what this means for India and the world
Jagadeesh Babu Rangisetty, Co-founder and Managing Director at Zenara Pharma, said, “We have launched this product in India with an aim to bring the best treatment options against COVID within reach of patients in our country. Our product, Paxzen, has been proven equivalent to Paxlovid through a Bio Equivalence study, based on which we have received the approval from the regulatory authorities.”
Zenara Pharma is a fully-owned subsidiary of the city-based Biophore India Pharmaceuticals. Pfizer’s Paxlovid has been approved by US FDA for COVID treatment.
With 6093 new cases detected in 24 hours by Friday noon, the active Covid-19 cases in India declined to 49,636, with the total tally rising to 4,44,84,729, according to the Union Health Ministry data.


