
- Home
- India
- World
- Premium
- THE FEDERAL SPECIAL
- Analysis
- States
- Perspective
- Videos
- Sports
- Education
- Entertainment
- Elections
- Features
- Health
- Business
- Series
- In memoriam: Sheikh Mujibur Rahman
- Bishnoi's Men
- NEET TANGLE
- Economy Series
- Earth Day
- Kashmir’s Frozen Turbulence
- India@75
- The legend of Ramjanmabhoomi
- Liberalisation@30
- How to tame a dragon
- Celebrating biodiversity
- Farm Matters
- 50 days of solitude
- Bringing Migrants Home
- Budget 2020
- Jharkhand Votes
- The Federal Investigates
- The Federal Impact
- Vanishing Sand
- Gandhi @ 150
- Andhra Today
- Field report
- Operation Gulmarg
- Pandemic @1 Mn in India
- The Federal Year-End
- The Zero Year
- Science
- Brand studio
- Newsletter
- Elections 2024
- Events
- Home
- IndiaIndia
- World
- Analysis
- StatesStates
- PerspectivePerspective
- VideosVideos
- Sports
- Education
- Entertainment
- ElectionsElections
- Features
- Health
- BusinessBusiness
- Premium
- Loading...
Premium - Events
Why a Nobel laureate feels India is not doing enough for science

In 2009, Venkatraman Ramakrishnan shared the Nobel Prize in Chemistry with Thomas Steitz and Ada Yonath for studies of the structure and function of the ribosome. The discovery of the double helix (the twisted-ladder structure of deoxyribonucleic acid) in 1953 by James Watson and Francis Crick was a turning point in the history of science. It helped scientists understand the detailed structure...
In 2009, Venkatraman Ramakrishnan shared the Nobel Prize in Chemistry with Thomas Steitz and Ada Yonath for studies of the structure and function of the ribosome. The discovery of the double helix (the twisted-ladder structure of deoxyribonucleic acid) in 1953 by James Watson and Francis Crick was a turning point in the history of science. It helped scientists understand the detailed structure of DNA, particularly on how it functioned when it came to transmitting and replicating genetic information. The discovery of the ribosome and its role in many proteins by George E Palade in 1955 was also significant. But the ribosome was not a simple molecule like the DNA. It was enormous and complex. Even though the ribosome had been studied for a couple of decades, nobody even knew where all the proteins in it were located.
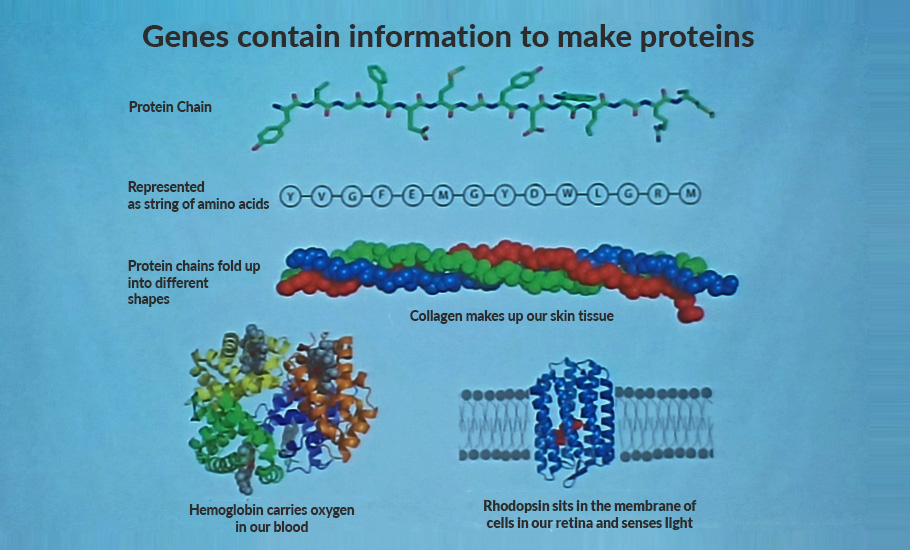
Ramakrishnan chose ribosomes as translation of genetic information into protein is fundamental to life. The ribosome, according to him, is the large macromolecular machine that translates the genetic code into protein in all life forms. An organism’s vital functions are managed by large, complex protein molecules produced in cells’ ribosomes. There, genetic information from messenger RNA is translated into chains of amino acids that then build proteins. Ramakrishnan’s contribution was that he was able to collaborate to map the structure of ribosomes, made up of hundreds of thousands of atoms, by using x-ray crystallography.
Born in Tamil Nadu’s Chidambaram in 1952, Ramakrishnan moved to Baroda (Vadodara) with his scientist parents when he was barely three years old. When he was 19, he left for University of Ohio to do his PhD. He spent five years there. The most productive thing that he did during this time was to meet Vera Rosenberry and marry her. What then led Ramakrishnan to ribosome?
It was a long journey from physics to biology. Having a PhD in physics, he left the subject and started learning biology. “Physics was a very mature field and it was difficult to make breakthroughs in it unlike biology. I thought of a transition, from physics to biology. Many scientists like Max Perutz, Francis Crick and Max Delbruck had made such transitions. So, I decided to go to a graduate school and learn the basics of biology. And by that time, I had just been married only a year earlier. And I had a stepdaughter who was six years old, and my son was born and he was only six weeks old,” said Ramakrishnan, who was in Chennai to launch the Tamil version of his book Gene Machine: The Race to Decipher the Secrets of the Ribosome, at the Asian College of Journalism in Chennai recently.

For Ramakrishnan, the journey was long, shuttling between the universities and labs in the US and the UK. Even though India bestowed on this Nobel laureate the prestigious Padma Vibhushan in 2010, the country didn’t contribute much to Ramakrishnan’s life as a scientist. The scientists in him grew up and developed in countries like the US and the UK. He visited India only three times in about 28 years since he left the country at the age of 19. He said he barely had any connection with Indian science during that time. It shows where India stands when it comes to scientific progress and achievements.
“If India wants to thrive as a country, it should invest more in science. Fifty years ago, China’s science was probably not as good as Indian science. But today, China is a global power in science. It has spent an increasing fraction of its GDP on science research and infrastructure development over the last 50 years. The government of India is not doing enough to promote science at all,” said Ramakrishnan, who was president of the Royal Society between 2015 and 2020.
At the same time, Ramakrishnan said, India had produced great scientists such as CV Raman, JC Bose and SN Bose to name a few before Independence. There was a tradition of excellence. After Independence, science got importance because the then Prime Minister Jawaharlal Nehru was interested in it. He funded a number of science institutions in the country. But after Nehru, science stagnated for a while, then, thanks to people like CNR Rao, and others, things started to improve again. But there was a problem. “When I grew up, the universities were fairly good in science. At least two professors in each department were doing some great work. The universities combined education and research in the same institution. Over the next two or three decades, the focus shifted from universities to the Central institutes. The central institutes eventually became the centre of all actions. This resulted in isolation of undergraduates and PhD students of various universities across India,” said Ramakrishnan, who is a structural biologist.
If he hadn’t gone to the US and stayed back, could he have had the same levels of achievements? “No,” he said. “India lacks labs and other infrastructure. Countries like the US, the UK and China, offer better facilities for research,” he said. Lack of funding, according to Ramakrishnan, is a major problem, but there are other factors that hold science back in India. “The dance between politics and science is an old one. It takes a lot of time to get anything approved and you have all sorts of restrictions from the bureaucracy on how the fund can be utilised. And even with the money it takes a long time and clearances are needed for each and every thing. The long process delays things. So, there are all kinds of drawbacks which hold science back in India,” he said. “India is not going to thrive based on its ‘cheap labour’. A country’s real progress is based on its knowledge-based economy,” he added.
Even though Scottish scientist Alexander Fleming discovered penicillin in 1928, it was the British government which supported clinical experiments of it at University of Oxford, eventually developing it to a modern antibiotic. While saying so, Ramakrishnan also said that the government can’t provide everything so the scientists should come forward and take the responsibility. “Scientists should not live in ivory towers. There should be public engagement. They should go to schools, colleges and public places and interact with students and people. They have to engage with the public so that the taxpayers will know the importance of science and they will support the initiative,” said Ramakrishnan. “The government should invest heavily on antibiotic research and that’s the only way forward. It should not let private companies alone do that job.”
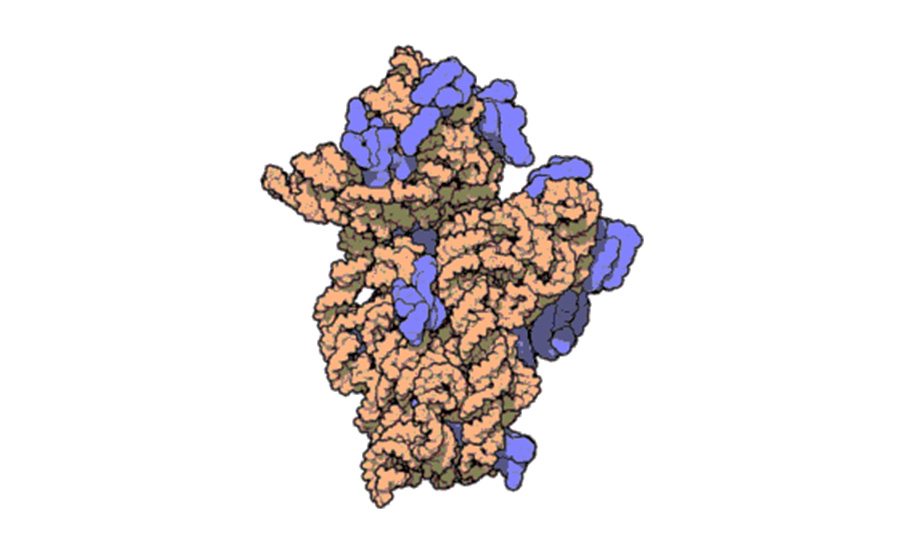
Ramakrishnan’s work on the elucidation of atomic structures of ribosomes helped us understand the structure of ribosomes in detail. He elucidated the atomic structure of 30S ribosomal subunit and subsequently shed light on the other structures of the entire ribosome in altered shifts and in complexes with several antibiotics. He has also been using electron microscopy to visualise ribosomes in action in higher organisms.
Ramakrishnan said he started studying ribosomes as a postdoctoral fellow in Peter Moore’s laboratory in 1978 where he participated in a project to determine the spatial location of the 30S proteins by neutron scattering, which culminated in the so-called “neutron map”.
“In 1983, I joined the Biology Department at Brookhaven National Laboratory as a staff scientist, and just two years later, senior scientist Stephen White, who had moved from Wittmann’s department at the Max Planck Institute for Molecular Genetics in Berlin joined me as a colleague. He had brought with him several crystallisation projects of individual ribosomal proteins. I decided to use the then new, more highly regulated version of the T7 expression system, which allowed us to clone the genes for and overexpress many of these proteins,” he said.
The result was that after a long hiatus, the pace of determination of structures of ribosomal proteins increased quickly. “It eventually became clear that these structures by themselves would not be ultimately useful in understanding ribosome function, even when used as labelling markers for footprinting studies or in modelling into electron microscopy maps of the ribosome,” he added. The duo then looked for the high-resolution structures of entire ribosomal subunits or the whole ribosome.
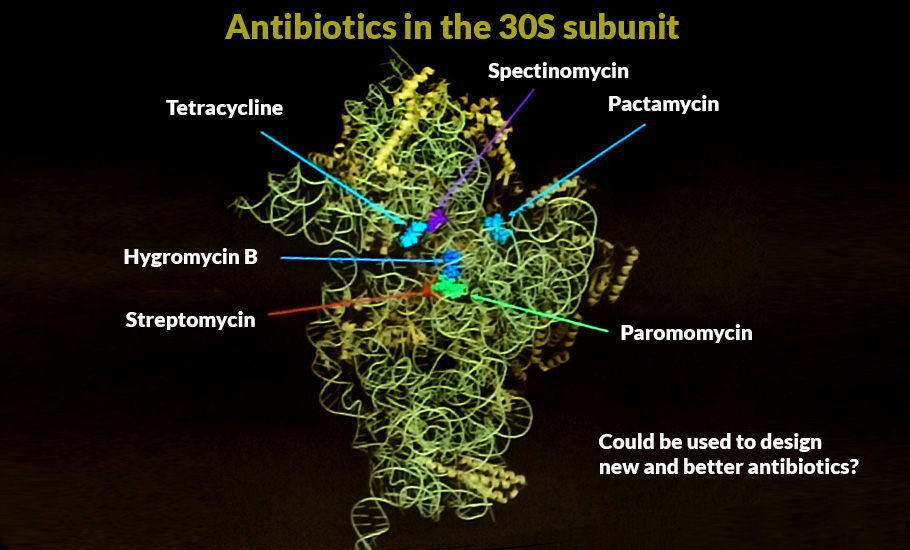
Ramakrishnan said the success in the determination of the high-resolution structures of ribosomal subunits and eventually the whole ribosome was the culmination of decades of effort. “In 2000, we solved the structure of the 30S ribosomal subunit and its complex with several antibiotics as well as its mRNA and tRNA ligands. Since then, we have solved the high-resolution structures of the entire ribosome at several points along the translation pathway, leading to insights into decoding, translocation and termination. We are currently working on translational initiation in both bacteria and eukaryotes, as well as how certain viral sequences disrupt the process in eukaryotes,” said Ramakrishnan, who works at the Medical Research Council Laboratory of Molecular Biology on the Cambridge Biomedical Campus in the UK.
