
- Home
- India
- World
- Premium
- THE FEDERAL SPECIAL
- Analysis
- States
- Perspective
- Videos
- Sports
- Education
- Entertainment
- Elections
- Features
- Health
- Business
- Series
- In memoriam: Sheikh Mujibur Rahman
- Bishnoi's Men
- NEET TANGLE
- Economy Series
- Earth Day
- Kashmir’s Frozen Turbulence
- India@75
- The legend of Ramjanmabhoomi
- Liberalisation@30
- How to tame a dragon
- Celebrating biodiversity
- Farm Matters
- 50 days of solitude
- Bringing Migrants Home
- Budget 2020
- Jharkhand Votes
- The Federal Investigates
- The Federal Impact
- Vanishing Sand
- Gandhi @ 150
- Andhra Today
- Field report
- Operation Gulmarg
- Pandemic @1 Mn in India
- The Federal Year-End
- The Zero Year
- Science
- Brand studio
- Newsletter
- Elections 2024
- Events
- Home
- IndiaIndia
- World
- Analysis
- StatesStates
- PerspectivePerspective
- VideosVideos
- Sports
- Education
- Entertainment
- ElectionsElections
- Features
- Health
- BusinessBusiness
- Premium
- Loading...
Premium - Events
How do egg cells grow? This experiment cracked the code
An enigmatic physical phenomenon, called ‘interconnected inflated balloon’, analogous to the exchange of gases between balloons of different sizes, appears to be the critical mechanism of the egg cells ending up big.

There is more to an egg than an omelette. An egg is a cell. It is made up of just one cell. Even an ostrich egg, typically weighing about 1.6 kg, is just a single cell. For most vertebrates, the egg cell is the most giant cell of all. Why the egg cells are enormous, we have some thought. Once fertilised, eggs have to support the growth of the embryo without external materials....
There is more to an egg than an omelette. An egg is a cell. It is made up of just one cell. Even an ostrich egg, typically weighing about 1.6 kg, is just a single cell. For most vertebrates, the egg cell is the most giant cell of all.
Why the egg cells are enormous, we have some thought. Once fertilised, eggs have to support the growth of the embryo without external materials. Nevertheless, biologists have not been clear how the egg cells end up becoming so big.
Led by biology postdoc Jasmin Imran Alsous and physics graduate student Nicolas Romeo, a team of Massachusetts Institute of Technology (MIT) biologists and mathematical physicist joined together to probe this riddle. Investigating the formation of the oocyte, immature egg cell in fruit flies, their research has revealed the intricate mechanism behind the growth for the first time.
They have confirmed the long-suspected role of the myosin, a molecular motor that gives traction to the cell. However, the action of the myosin alone does not provide a complete picture.

An enigmatic physical phenomenon, called ‘interconnected inflated balloon’, analogous to the exchange of gases between balloons of different sizes, appears to be the critical mechanism of the egg cells ending up big.
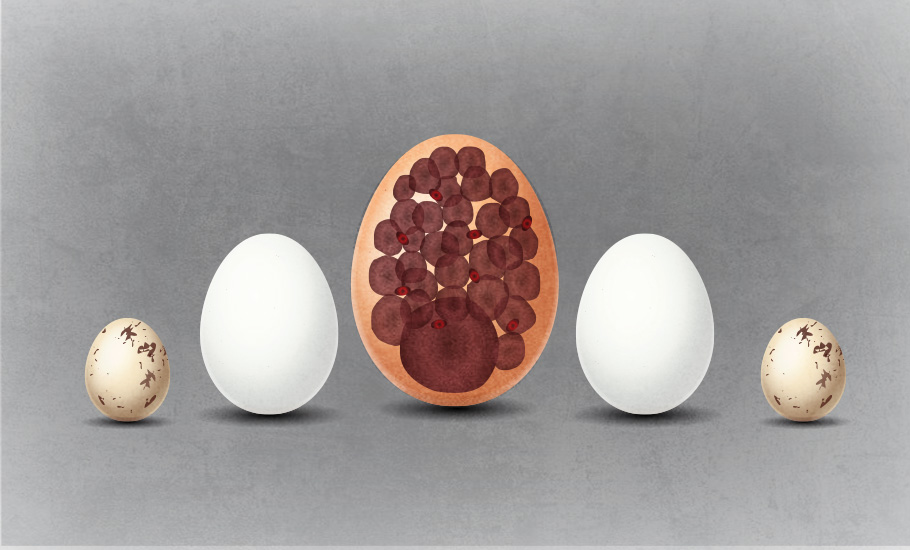
Enormous egg
Ranging between 1100 and 1950 grams, the ostrich egg is the largest egg of any living bird. The bee hummingbird produces the smallest known bird egg among birds, weighing just half of a gram. Of course, fish and other organism have even more microscopic eggs. Even the egg cell is typically 200 micrometres, and are massive compared to other cells. In contrast, the human sperm cell is 10,000 times smaller.
The eggs of the now-extinct elephant birds, flightless birds that once thriving thousands of years ago on the island of Madagascar, are the largest known. One elephant bird egg weighed 10 kg and is 160 times larger in terms of volume compared to a typical chicken egg.
The ostrich may lay the world’s largest bird’s egg but the female kiwi lays a bigger egg than almost any other bird in proportion to its body size. While an ostrich egg is about 2% of the bodyweight of the mother bird, and a human baby at full term is 5% of the mother’s body weight, the kiwi’s egg is whopping 20% of the bodyweight of the mother bird.
How the egg cell becomes big
In some animals like frogs, eggs are expelled before fertilisation. The fertilisation occurs outside the mother. In some animals like birds, the mature eggs are fertilised before they are ejected. In some animals like snakes, the fertilised eggs are detained until they hatch.
Starkly distinct, among mammals, the growing embryo is retained until it turns into a young animal, as a larva or newborn, before it is released. Irrespective of the manner, the growth of the immature egg cell, oocyte, is a crucial step in reproduction.
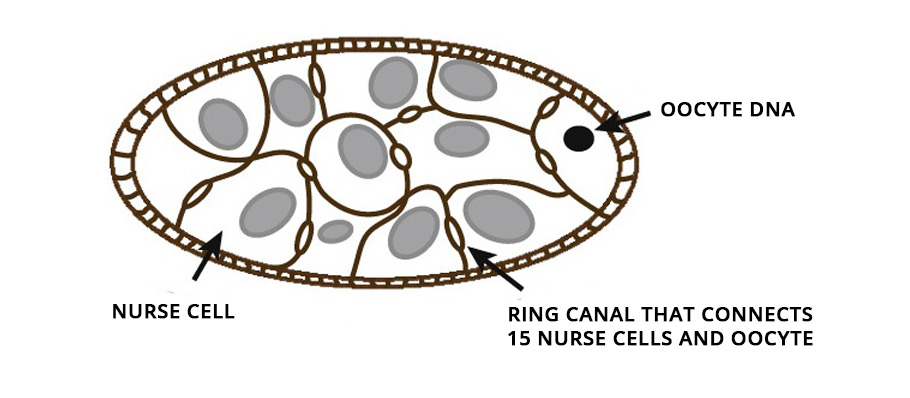
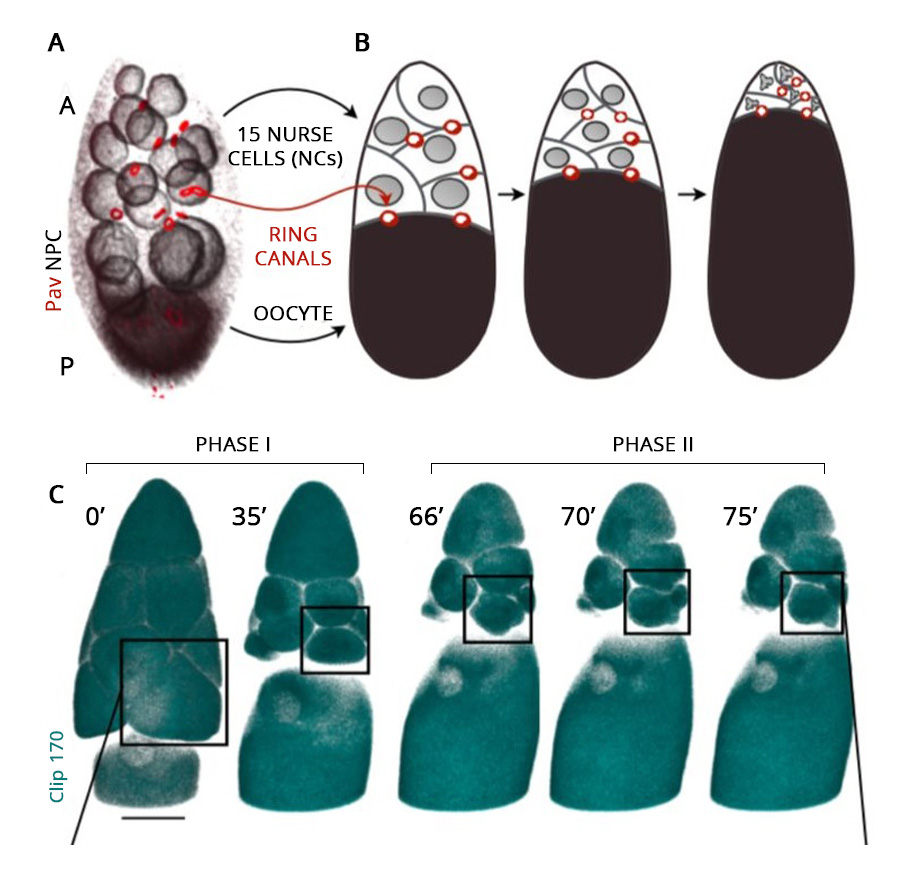
From insects to mice, oocytes develop within a cell cluster known as cysts. In female fruit flies, for example, in the first step, the immature oocyte undergoes cell division and produces 16 cells. During the cell division, the cell separation remains incomplete and stays connected with the adjacent cells, permitting the movement of cytoplasm from one to another.
Of these 16 cells in the cysts, the bigger one ultimately develops into an oocyte. The remaining 15 nurse its growth by dumping their cytoplasmic contents into it. On accumulating additional cytoplasmic flow, the developing oocyte cell inflates and grows in size. Ultimately the nursing cells, left with nothing but their nucleus, regress and die. The immature oocyte mellows into a gigantic egg cell.
How the oocyte cells grow was familiar but the mechanism that drove it remained a riddle. How do nursing germ cells in a cyst transport the entirety of their cytoplasm into the future egg cell before undergoing cell death?
Live imaging
Using a Zeiss LSM 710 point-scanning confocal microscope, researchers employed a live imaging technique to capture the process frame by frame. The high-resolution imaging with 400 milliseconds and 900 milliseconds stop-motion video were obtained. The entire process of oocyte growth, lasting about three hours, was captured.
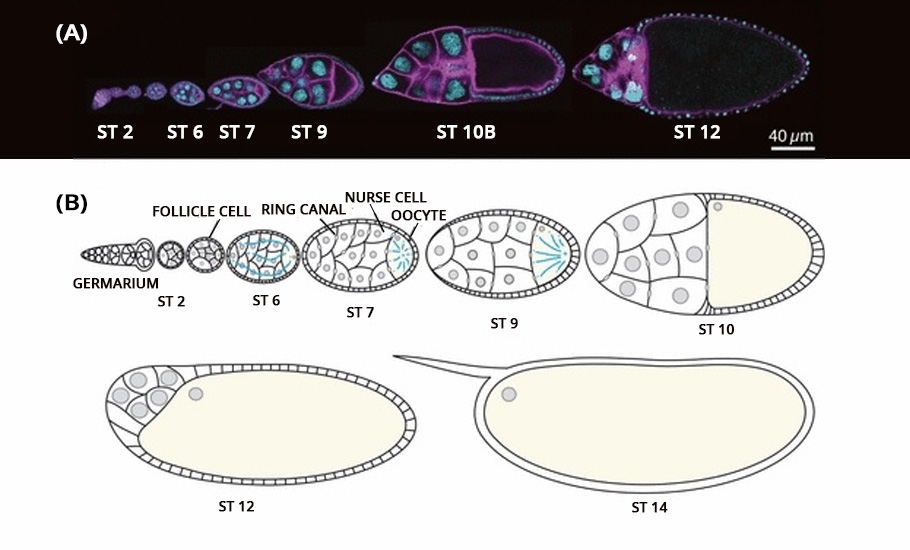
The live video amazed the researchers. The oocyte was the largest cell in the egg chamber. The nursing cells were arranged in four layers exhibiting a descending cell size order according to their distance from the oocyte.
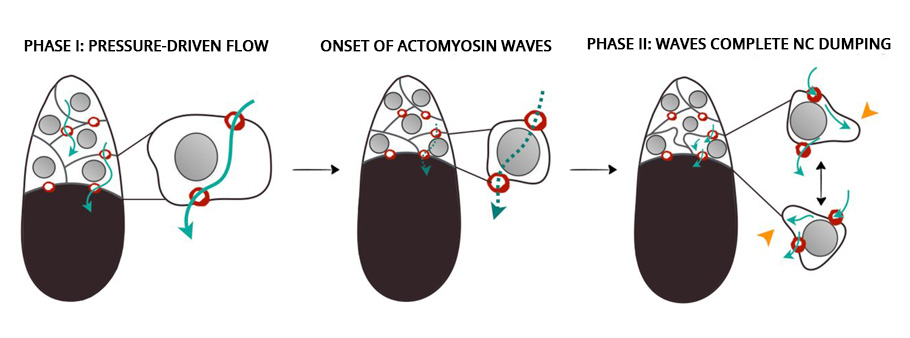
The development of the oocyte cell clearly had two phases. In the first phase, the nursing cells, one by one, dumped their cytoplasmic contents into the developing oocyte cell. The data showed that the nursing cells in the first layer, which are also the largest and directly connected to the oocyte, were the first to transport their contents into the oocyte, followed by the smaller nursing cells in other layers. Once the nursing cells emptied 75% of their contents, the first phase came to an end.
In the second phase of the oocyte development, the nursing cells experienced a wave-like movement, a telltale sign of the myosin initiated pulsating contraction. The onset of the actomyosin waves in the nurse cells closer to the oocyte occurred earlier before spreading to nursing cells in the outer layers.
Compressed by the action of the myosin, the self-contracting cells expelled the remaining cytoplasmic materials into the oocyte cell. At the end, devoid of any cytoplasmic materials, the nursing cells disintegrated and one giant hefty oocyte cell remained.
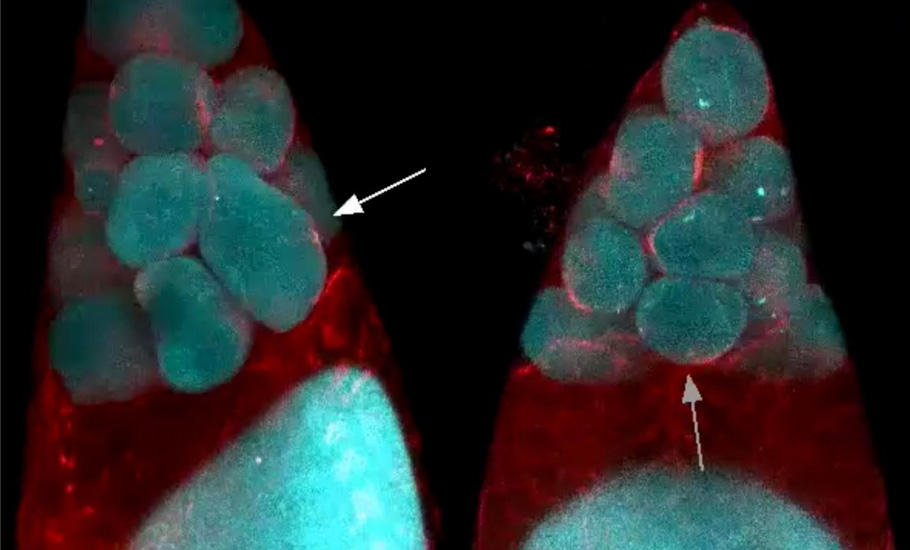
Myosin motor
Myosin is a protein that converts chemical energy to mechanical energy, generating force and locomotion in the cells. From stretching our hand to the ticking of the heart, it is the myosin motor drives the motion.
The myosin molecular motor is behind the ability of the white blood cells to crawl towards a wound site and the macrophages to hunt the invading germs. Like an electrical motor that gives traction, the sliding interaction of actin and myosin filaments, called actomyosin, generate contractile forces.
Developmental biologists hypothesised that the contractile forces generated by actomyosin powers the nursing cell dumping. To test the hypothesis, the researchers knocked out the myosin during the oocyte maturation. If the myosin was powering the transfer, the nursing cells must not empty their content without it.
Astonishingly, even in the absence of myosin, the first phase of the oocyte development took place unhindered. However, the wave-like contractions, the second phase, did not take place. The unexpected twist put the researchers in a spot. This clearly showed that a mechanism other than “squeezing” initiated the transport during the first phase.
Two balloon experiment
To fully understand the process, the biologist team sought collaboration with the mathematical physics group at MIT. The researchers, Jorn Dunkel and his PhD student Nicolas Romeo, studying physics of soft surfaces and flowing matter, wondered, looking at the inflating oocyte video, whether the solution is concealed in the famous two-balloon experiment.
In the experiment, we take two identical balloons and inflate them, one big and the other smaller and connect both the balloons using a tube and a clamp in between. If the clamp is released to let air flow freely between the two, what do you think will happen? Bewilderingly, belying expectations, the smaller balloon empties its contents into the larger balloon, resulting in the smaller balloon becoming even smaller and the bigger one expanding.
This seemingly counterintuitive phenomenon results from the Young-Laplace law. The smaller the inflated balloon, the greater the curvature, and hence, there is more surface tension, leading to a higher surface pressure. Therefore, for a sufficiently large balloon, the force on the surface is inversely proportional to its radius. Accordingly, a bigger balloon will have a lower pressure at its surface than the smaller one, driving the air to flow from the smaller to the bigger.
Hydraulics in the cyst
Extending the two-balloon problem and representing the oocyte cyst with sixteen cells as a network of sixteen interconnected balloons, the researchers modelled the mechanism behind the first phase of oocyte growth.
This got the researchers thinking whether this networked fluid flow model explained the intercellular cytoplasmic transport during the first phase. In that case, it must exhibit a specific spatio-temporal pattern. The dumping must unfold in a hierarchical manner that mirrors the nurse cell size and arrangement.
The video of the cyst development showed that the nurse cells directly connected to the oocyte were the first to transport their contents into the oocyte, followed in order by smaller nursing cells located further away.
Further, the model also predicts the backflow of the material during the dumping. Say, three balloons, A, B, C, each with decreasing size in order, A being the biggest and C being the smallest, are interconnected. Once the clamp is removed, B will initially dump its content into A and become even smaller than C. Now, as the B is the smallest, its content will flow towards C, making it slightly bigger. At this stage, there will be a backflow of the material away from A. Indeed, as predicted by the networked fluid flow model, such transient backflow away from the oocyte was observed during the nurse cell dumping, giving confidence that the model is robust.
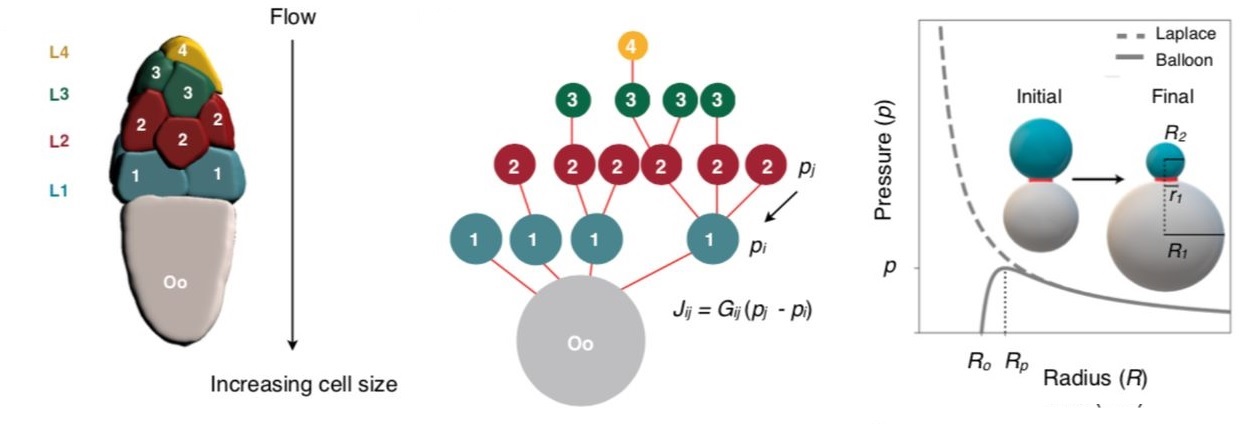
“The flow-network model robustly captures qualitatively essential features of the experimentally observed transport dynamics… Specifically, the model correctly predicts both the hierarchical pattern of intercellular transport and timescale of nurse cell dumping…. proposed model successfully captures experimentally observed complex transport patterns along the 16-cell tree,” say the researchers.
The quest continues
“Science never solves a problem without creating ten more”, famously said George Bernard Shaw.
While this study has illuminated how phase one of oocyte growth occurs, several questions still remain unanswered. What triggers the second, myosin-powered phase of the oocyte growth? Will the change in the original sizes of the nurse cells affect the formation of the egg cell? In mice, for example, the oocyte develops as a cyst with other interconnected cells. However, can one generalise and assume if this exact mechanism operates in all mammals?
