
- Home
- India
- World
- Premium
- THE FEDERAL SPECIAL
- Analysis
- States
- Perspective
- Videos
- Sports
- Education
- Entertainment
- Elections
- Features
- Health
- Business
- Series
- In memoriam: Sheikh Mujibur Rahman
- Bishnoi's Men
- NEET TANGLE
- Economy Series
- Earth Day
- Kashmir’s Frozen Turbulence
- India@75
- The legend of Ramjanmabhoomi
- Liberalisation@30
- How to tame a dragon
- Celebrating biodiversity
- Farm Matters
- 50 days of solitude
- Bringing Migrants Home
- Budget 2020
- Jharkhand Votes
- The Federal Investigates
- The Federal Impact
- Vanishing Sand
- Gandhi @ 150
- Andhra Today
- Field report
- Operation Gulmarg
- Pandemic @1 Mn in India
- The Federal Year-End
- The Zero Year
- Science
- Brand studio
- Newsletter
- Elections 2024
- Events
- Home
- IndiaIndia
- World
- Analysis
- StatesStates
- PerspectivePerspective
- VideosVideos
- Sports
- Education
- Entertainment
- ElectionsElections
- Features
- Health
- BusinessBusiness
- Premium
- Loading...
Premium - Events
COVID-19 vaccine soon? Why you need to keep the mask on for now
Bharat Biotech’s Covaxin, an inactivated virus vaccine for novel coronavirus, is a sweet respite coming amid the rise in COVID-19 cases in India.

With COVID-19 cases touching 6 lakhs and counting, the unanticipated but pleasant announcement by the Bharat Biotech of COVAXIN, an inactivated virus vaccine for novel coronavirus, came as a sweet respite. In the widely reported letter, the Director-General of the Indian Council for Medical Research (ICMR), has directed everyone to speed up the clinical trials. How does a vaccine work? What...
With COVID-19 cases touching 6 lakhs and counting, the unanticipated but pleasant announcement by the Bharat Biotech of COVAXIN, an inactivated virus vaccine for novel coronavirus, came as a sweet respite. In the widely reported letter, the Director-General of the Indian Council for Medical Research (ICMR), has directed everyone to speed up the clinical trials.
How does a vaccine work? What is inactivated virus? How is it developed? At what stage are we in the development process? Realistically, how long will it take for the vaccine to be developed and deployed for public use.
In simple terms, an effective vaccine is a trigger that trains our ‘immune system’ to recognise the pathogen, say novel coronavirus, and produce appropriate antibodies and T-cells that can neutralise the virus when the infection actually occurs.
Antigen and antibodies
The pathogens have body parts with specific molecular structures, called antigens, which induce the immune system response. The immune system of the human body develops antibodies against an invading disease-causing virus. The subset of antibodies neutralise virus infectivity and prevent further infection. One can imagine the antigen as a ‘nut’ and the Y shaped antibody as a spanner. To unfasten the nut from the bolt, the wrench has to fit and grip the nut well.
Most nuts have a hexagonal head, but there are cap nuts, barrel nuts, cage nuts, wing nuts and so on. They also come in various sizes. We also have matching spanners for each of these nuts—open-end wrench, box-end wrench, swivel head spanner, socket wrench and so on. Only with the paired spanner we can unfasten the nut, likewise, only a matching antibody can bind the antigen and deactivate the pathogen.
While you might have just one or two types of a spanner at your disposal, a car mechanic will have tens of hundred. Researchers say we are born with thousand crores (10 billion) different antibody molecules as part of our immune system antibody repertoire. However, often the pre-existing ‘spanner’ antibodies are no match for a new germ in the block, mainly when it is a newly evolved virus, like novel coronavirus.
Made for each other
If the right fit spanner is not available, the mechanic does not give up but try to wind twine around a wrench to make it grip the nut. Like manner, when a hitherto unknown pathogen invades, near fit antibodies from the repertoire of crores of antibodies of the immune system already available would make an effort. However, unlike a wrench, the antibody, a biological substance can evolve.
While the bottom portion, the single vertical dash of the Y shaped antibody remains unchanged, one side of the tips of the two forked parts can adapt and evolve. With multiple cycles of development the antibody matures and binds better with the target antigen. The subset of antibodies that are capable of neutralising the infectivity of a particular virus are called neutralising antibodies. Such antibodies are specific to a particular pathogen. Often they are ineffective against another germ.
Forget not
Antibody development is not fire and forget. Most computers come with star-shaped Torx screws which need a Torx key to open. Once we buy one, it is not thrown away. It is put away for future use and gathers dust in the toolbox. Likewise, once the immune system develops a particular antibody for a pathogen, it never forgets. The memory of the antigen and the antibody is ‘saved’ for posterity in the immunological memory B cells and T cells. When the same pathogen attacks next time, no time is lost in adapting and developing the right fit antibody. The memory cells are jostled, and the torrent of matching antibodies are produced. In the ensuing blitz, the pathogen has no chance. This is why most viral diseases, say chickenpox, do not strike twice. We acquire immunity for that pathogen.
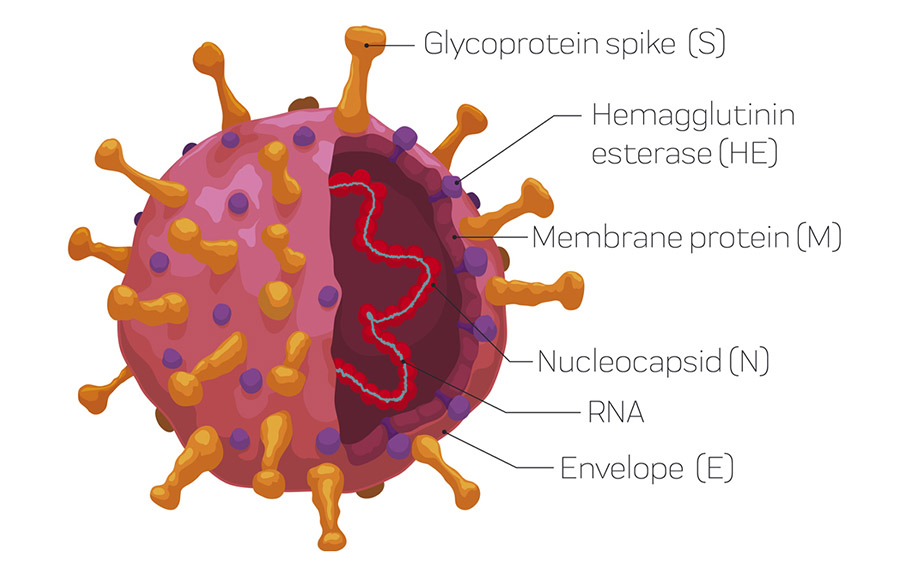
Novel coronavirus has a simple structure. Its genome, a small RNA segment, is scaffolded around nucleocapsid N protein. This is wrapped with three proteins, envelope (E), spike (S), membrane (M) proteins. The spike protein consists of S1 and S2 domains. A portion of the S1 is the receptor-binding domain which latches to the surface of the human cells resulting in infection. The S2 domain, the receptor-binding domain of S1, nucleocapsid protein can perform the function of the antigen. The immune system produces antibodies against each of these antigens.
Tug of war
Before the antibody maturation, the novel coronavirus may have the upper hand. It could spread unchecked and ravage the lungs. It can perhaps breach and reach the blood, travel to the heart, brain, and whatnot. Once the neutralising antibody is produced, then it is a tug of war between the pathogen and immune system.
Perhaps you are old, your immune response will be sluggish. Maybe you are economically poor and undernourished; the immunity is enfeebled. Or you have co-morbidity like blood pressure or diabetes. Then the virus has already spread like wildfire. Your lungs, blood vessels are ruined, and perhaps some tissues have died for want of oxygen before the immune system could galvanise the neutralising antibody. The balance in the tug of war is precarious. Many recover, some die.
On the other hand, you are young, well-nourished and not suffering from co-morbid conditions, your immune system usually is agile and swift. You can pull it off and survive. Meanwhile, if you have not been practising COVID19 hygiene, face mask, hand washing and physical distancing, then you would have gifted the virus to others.
Life and death, ultimately depend upon the response time. How fast the immune system is triggered? How swift it can produce the neutralising antibody?
How to train the immune system
All it takes for a sniffer dog is smell the culprits’ clothes. Once the dog recognises the scent, it leads you along the path taken by the criminal. The immune system can also be trained to identify a particular germ before an actual infection. This is vaccination.
From school laboratories to natural history museums, you must have seen snakes, frogs and other animals inside glass vials preserved pristine with formaldehyde. The animals are dead, but the body does not decay. We can inactivate a virus by applying formaldehyde. The virus is ‘killed’,but the key body parts are still intact. One can also use heat to inactivate the virus. Once the viruses are entirely inactivated, it cannot possibly reproduce itself or cause disease. However, critical structural elements of proteins that function as antigens are uninjured. In plain English, an inactivated virus is the dead but intact body of the virus. Remember, virus is neither living nor non-living. Hence one cannot talk of ‘killing’ a virus, that is why the jargon ‘inactivated’.
The whole organism, alive and kicking, is not at all required to trigger the immune response. What we really need is the antigens, intact ‘nuts’, on the surface of the pathogen. Once the immune system recognises an antigen, it goes literally nuts. Deceived into thinking an attack by a hitherto unknown invading pathogen, the immune system is kicked into action. The immunological response is unleashed, and the antigen-specific antibodies are generated, along with memory. Lo behold we have acquired induced immunity. Now the pathogen cannot infect us.
Road to development
To produce an inactivated virus, first, you have to grow them in cells in a petri dish. Then the virus has to be inactivated. At times intact proteins of the virus can be toxic. We have to ensure that the inactivated virus is safe. What if the process of inactivation has destroyed key antigen structures. Then the right kind of antibodies will not be generated. One has to test the efficacy in a laboratory before conducting animal studies. The National Institute of Virology in Pune, an institute under the ICMR network isolated a virus from an Indian patient and successfully cultured in a laboratory conditions. This was a crucial step.
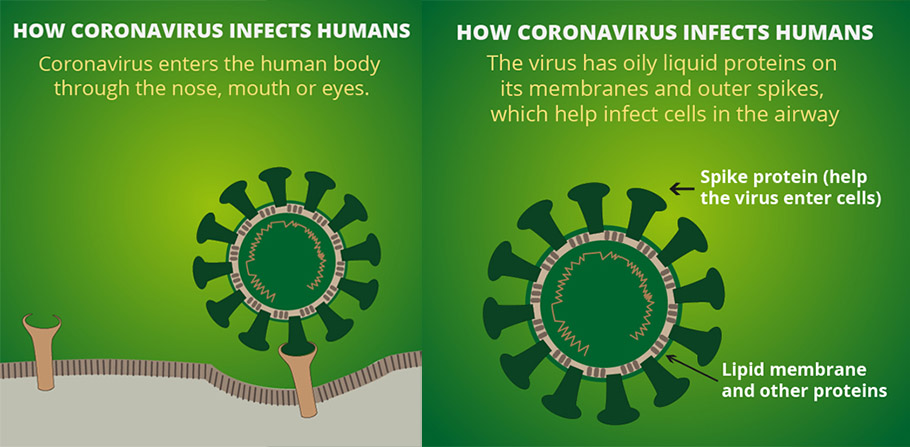
Growing the novel coronavirus in a lab is not that easy. The novel coronavirus is a snob. It can infect only certain types of human cells; the human airway epithelial cells, that is cells that form the inner lining of the respiratory passage. That is why we get infected only with nose, mouth or eyes. The epithelial cells are rich in a receptor called ‘ACE2’, and the novel coronavirus binds to these receptors to gain a foothold in human cells. The skin cells, for example, lack this ACE2 receptor and hence this virus cannot infect clean human skin cells. The virus is cultured in the laboratory in the human airway epithelial cells or Vero E6 cells isolated from kidney epithelial cells of an African green monkey.
The next challenge is to test the safety and efficacy of inactivated virus in animals. The trouble is novel coronavirus love to infect humans and cannot infect, say rats; the reason is the lack of ACE2 receptors in rats. A transgenic mouse which has ACE2 receptors in its airway epithelial cells was developed. With the ACE2 receptors in the airway, these hACE2 experimental mice are now susceptible to infection by the novel coronavirus like humans. The efficacy and safety of the vaccine will be first tested on these hACE2 mice before human trials are commenced. These are some of the important steps of pre-clinical trials. Then comes the human trials.
What next?
COVAXIN, we are told, is entering the phase I clinical trial. The ICMR has asked twelve COVID hospitals to fast track clinical trials of the vaccine. This implies that the pre-clinical trials, including animal trials, must have been completed, although the information related to this is not available in public domain.
In the human trial phase I, a set of healthy humans will be administered with the vaccine to test the safety. Their vital organs will be monitored, and blood samples examined regularly. The phase I trial will tell us if it is safe for use, and if the immune response is triggered. That is if the vaccine can artificially induce immunity against the novel coronavirus.
If the phase I is successful, then the phase II clinical trial with participation of several hundred, including people at risk has to be undertaken. One-half of the people selected for the examination will be administered the test vaccine. At the same time, the other half will receive a placebo. In this randomised, double-blind trial, both groups will be observed to see how many of them contract the disease to assess the efficacy of the vaccine.
The public information available in the Clinical Trial Registry of India (CTRI) indicates that the follow ups of the trial will be carried on Day 14, Day 28, Day 104 and Day 194, that is about six months. The whole effort may take a year and three months according to the submission made by Bharat Biotech.
This is often followed by Phase III trials which will involve an even more significant number of participants, including the susceptible population. If the trial is a success, then the firm is given a licence to manufacture and sell the vaccine. Nonetheless, large scale surveillance of select people among those who have been administered the vaccine will continue to be undertaken. Called the clinical trial IV this will be conducted for many years, to detect any long term adverse effect.
