
RT-PCR tests: Researchers devise field deployable alternative

Researchers from the Indian Institute of Science (IISc), Bangalore, have devised an alternative method to RT-PCR, considered the standard test for detecting viruses like Ebola and SARS-CoV-2.
The newly developed ‘Recombinase Polymerase Amplification (RPA)’ method, wherein all the reactions take place close to room temperature, does not require a thermal cycler. The process can be employed for robust and sensitive nucleic acid estimation, and can be beneficial for field deployment in resource-limited settings.
In Real-Time Polymerase Chain Reaction (RT-PCR, also known as quantitative PCR or qPCR) method, a throat swab or a blood sample is taken from the patient who may have only a few viral DNA or RNA molecules. This makes the detection of the virus quite tricky. Therefore, the number of DNA or RNA molecules is to be amplified using PCR.
Also Read: Earth’s water could be older than the Sun: Study
“The newly generated DNA is tagged by fluorescent molecules and monitored in real-time to estimate the level of DNA (or viral RNA) in the sample. This technique also uses a thermal cycler which regulates the temperature because different temperatures are required in multiplying the DNA or RNA molecules. However, the use of thermal cyclers and real-time monitoring limits the use of RT-PCR in resource-starved areas,” reads the report published on the IISc website.
Amplification-based quantitative polymerase chain reaction (qPCR) provides accurate and sensitive nucleic acid quantification. However, the temperature cycling and real-time monitoring requirement limits its translation to many settings.
To overcome this limitation, two researchers from the Department of Chemical Engineering, Priyanka Valloy and Rahul Roy, used an alternative technique called the Quantitative endpoint Recombinase Polymerase Amplification (qeRPA) to determine the initial number of DNA copies in blood samples from dengue patients.
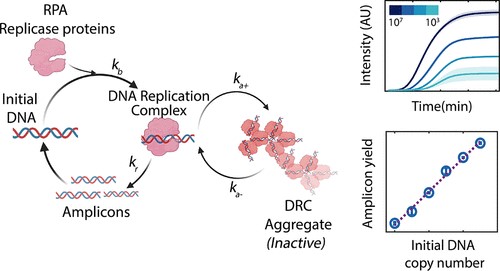
“The results from qeRPA were consistent with the results obtained from qRT-PCR while being faster and easier to implement,” informed the researchers. Unlike the conventional isothermal quantitative methods, qeRPA can be employed for robust and sensitive nucleic acid estimation at close to room temperature without real-time monitoring and can be beneficial for field deployment in resource-limited settings.
They identified conditions that constrained the reaction yield corresponding to the starting DNA template concentration. After validating these predictions experimentally, they showed that the amplicon yields at the end of the RPA reaction correlated well with the starting DNA concentration while reducing nonspecific amplification robustly.
“Quantitative isothermal amplification methods alleviate the need for thermal cyclers; however, they still require continuous monitoring of the nucleic acid amplification on sophisticated readers. Here, we adapted an isothermal recombinase polymerase amplification (RPA) reaction to develop a semiquantitative method that relies on the final amplicon yield to estimate the initial target nucleic acid copy number,” the researchers explain.
Also Read: How fish evolved to walk — and in one case, turned into humans
This method can detect nucleic acids like DNA or RNA at diagnostic centres in resource-limited areas such as remote villages and developing countries, making diagnostics more accessible.
“We validated these predictions experimentally and showed that the amplicon yields at the end of the RPA reaction correlated well with the starting DNA concentration while reducing nonspecific amplification robustly”, write the researchers. They have published an article based on the study has ACS Publications’ journal of Analytical Chemistry.

