Cryo-electron microscopy: The game-changer for structural biology

It is said, a picture is worth a thousand words. What better if that picture is a ringside view of minute particles like viruses or functional proteins, along with changes they undergo? Such intricate, three-dimensional blueprints of biological samples are worth a library of information to scientists. Insights into the molecular structures are not only valuable in understanding how they function, but also help to detect the anomalies that arise of them.
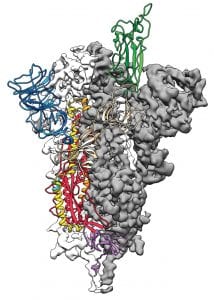
To cite an example, when the new viral infection broke out in China’s Wuhan, within weeks, scientists showed us how the virus looked like. The images of exquisite clarity revealed the architecture of the spike protein that the virus employed to invade human respiratory cells. The structural details divulged by these images are propelling several scientific studies to search for targeted therapies for COVID-19.
Are you wondering how such images are taken? Or what makes up such a tool, and the technology behind it? Then, hop on board! Let us explore a Cryogenic Electron Microscope (cryo-EM). A highly advanced instrument that is now helping scientists to ‘look’ at the atomic scale structural arrangements of biological specimen.
Better than the best
A Transmission Electron Microscope (TEM) is an imaging instrument which is similar in working to an optical microscope. The main difference is in the light source: Instead of visible light, TEM uses a high-energy electron beam radiation. Electrons have a smaller wavelength compared to light photons. They are, hence, capable of penetrating into the molecular framework and reveal the finer details.
Discovered in the 1930s by Ernst Ruska and Max Knolls, the TEM has steadily evolved over the decades. The present version — which is primarily used for Life Sciences — is called the Cryogenic Electron Microscope. The cryo-EM was developed by Richard Henderson, Joachim Frank and James Dubochet. The three scientists invested more than four arduous decades in refining the TEM into cryo-EM.
In 2017, they were rewarded with a Nobel Prize for their pioneering work, which is transforming structural biology. Their novel method of operating the microscope at very low temperatures makes cryo-EM one of the most powerful imaging instruments today. The tool is sought after for its versatility, high-resolution and the convenience of photographing the samples directly.

Cryo-EM is a significant alternative to traditional techniques like X-ray crystallography and NMR spectroscopy. It neither requires large quantities nor crystals of the samples for observation. Besides, the changing states of the molecules can also be photographed. While small –to- medium-sized samples work well with traditional techniques, for bigger particles, the Cryo-EM fairs better.
Embedded proteins (like neuro-receptors that lay hidden in cell membranes) or dynamic proteins (those that change shape, like the coronavirus spike protein) can readily be photographed using the Cryo method. The instrument is also useful to observe several biomolecules at a time.
Over the years, the use of ultra-fast detectors with high signal-to-noise ratio, improved automation, and sophisticated algorithms for image processing has enhanced the efficiency of the instrument. The improvisations enable extracting a higher number of images to reveal greater molecular depth. Cryo-EM is now employed to observe a spectrum of biological samples: Tissue sections, frozen samples, bacteria, viruses and a horde of functional proteins.
The building blocks
At the heart of cryo-EM are three units. An Electron gun generates an optimised beam of electrons. The radiation is then passed through a vacuum chamber (to avoid scattering). An accelerator impinges the beam on the specimen.
The next is an Imaging System comprising of a set of specialised lenses called electromagnetic lenses. The lenses collect the ‘shadow’ cast by the sample due to the bombarding electron beam and orient them towards the detector screen. Thus, they magnify and generate projection images. From here, the multiplied beam falls on the Detecting Unit, which is a high-speed digital camera. The camera captures the image of the molecules (and their different positions) in rapid succession and stores them.
All images with similar ‘shadows’ are aligned and integrated to get two-dimensional outlines of the molecules. Then, all similar molecules are ‘averaged’ to reduce the noise generated due to specimen damage induced by the impinging electrons. Commonly, the instrument takes several thousand such 2-D images to get a high-resolution.
The computational infrastructure that augments the microscope analyses the vast volume of 2-D image data and generates a spectacular 3-D representation of the specimen.
What makes it a ‘cryo’ microscope?
Cryo means ultra-low temperatures, and that is what the specimen is subjected to during cryo-microscopy. Here, samples are frozen to temperatures of -190 degree Celsius and photographed.
Sample preparation is vital before photographing it. For this, the specimen is combined with a compatible liquid (like water) and dropped on a metal grid. The fluid-specimen combination forms a thin- film around the holes in the grid. The grid is then immediately plunged into liquid nitrogen to freeze the specimen as-is.

The sudden cooling ‘vitrifies’ the liquid. In other words, under the temperature shock, the liquid molecules do not have enough time to crystallise. Instead, they freeze to form disordered glass-like structures, inside which, the specimen is trapped in its native form.
The vitrification is a crucial step to imaging, as electron beams are prone to diffract through crystals. All through the photographing stage, the sample is maintained at the cryo temperature to slow the onset of radiation damage to the organic molecules. Also, the plunge-freezing captures the molecules in the various stages of conformational changes they may be undergoing.
Revolutionising structural biology
With such groundbreaking technologies at hand, it is an exciting time for structural biologists, and Indian researchers have made significant contributions to this field. So far, of the 1000 unique molecules described globally by cryo-microscopy, India has contributed to 16.
The cryo-EM is a high-end instrument and costs around 60-70 crores, requiring expertise to set it up. The Bangalore Life Science Cluster, NCBS, Bengaluru, has an advanced facility that operates a 300kV unit to generate 0.02 Å wavelength electron beam. The instrument is capable of obtaining atomic resolution structures of macromolecules typically in the range 2-3.5 Å or better. Apart from here, IISc, Bengaluru, also has a Cryo-EM facility, and several more are likely to be established in the coming years.
(With technical inputs from Dr Vinothkumar Kutti Ragunath, National Centre for Biological Sciences, Bengaluru)

