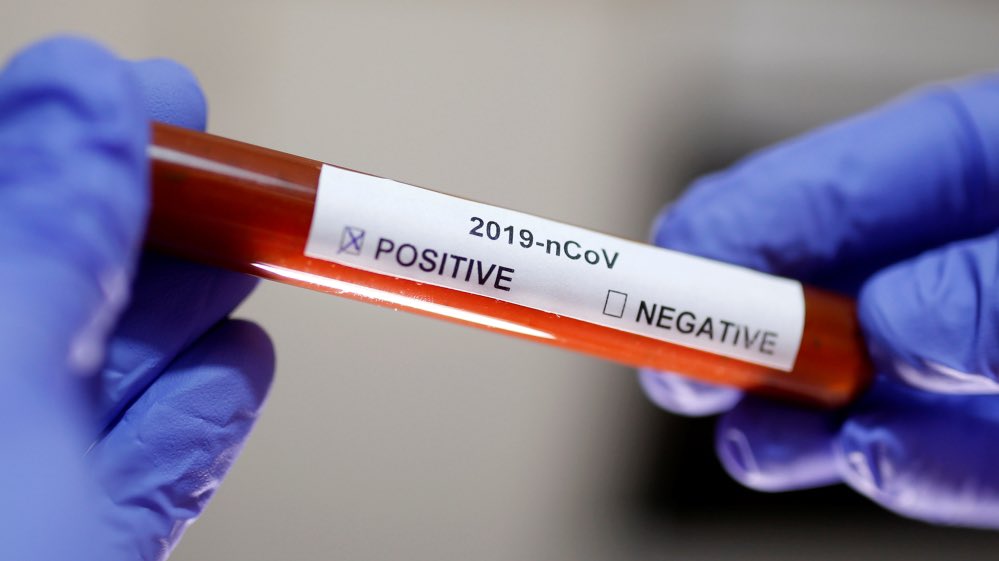
ICMR to consider biobank of test samples for COVID-19 research
The new test kit could help determine the efficiency of a future vaccine and guide the hospital discharge policy.

A task force set up by the apex medical research body, Indian Council of Medical Research (ICMR), has recommended launching a biobank or a repository of test samples to understand clinical patterns in COVID-19 cases.
The research will also comprise expediting test kit validation, and to study the immune response to coronavirus. While the samples are being discarded presently, the task force members monitoring diagnostics and biomarkers said that the agency has selected nine ICMR laboratories, where they can store positive and negative nasal, throat, and blood samples for research purpose, reported The Indian Express.
The laboratories selected have easy access to dedicated COVID-19 hospitals. The standard operating procedures for the research project is being laid out.
ICMR can record clinical, social, and demographic factors using the samples. The results can be utilized for cutting-edge research, including genome sequencing projects. ICMR has received several diagnostic test kits requiring validation. It has also received many medical supplies or products for validation, such as PPE fabric, gloves, masks, mobile applications, ayurveda formulas, herbal formulations, and hand sanitizers.
RELATED NEWS: ICMR asks states to survey COVID-19 exposure among vulnerable groups
The new test kit could help determine the efficiency of a future vaccine and guide the hospital discharge policy.
The commercial manufacturers of diagnostic kits and AYUSH regimes will send their products to the designated institutes. ICMR’s own centers will also validate diagnostic kits, viral transfer mediums, as well as RNA extraction kits. Along with the bio-repository, the task force by ICMR has also recommended validating a new antibody assay that can help determine the level of resistance a patient has against COVID-19 re-infection.

