Researchers find novel way to target Triple-Negative Breast Cancer

If we think of cancer cells as a house, the receptors are the locks of the house’s front door. Triple-Negative Breast Cancer (TNBC) is more challenging to handle as it does not express commonly found breast cancer receptors, e.g., Estrogen (ER) and Progesterone (PR). This leaves doctors with less options to destroy the cancer cells.
A team of researchers led by Dr Dipak Dutta, principal scientist at the Cancer Biology division of CSIR-Central Drug Research Institute (CDRI), Lucknow, has come up with a new and striking mechanism for EZH2-histone trimethylation-mediated gene upregulation instead of its classical suppressive function. Apart from Dr Dutta, the CDRI researchers team comprised Ayushi Verma, Akhilesh Singh, Manish Pratap Singh, Mushtaq Ahmad Nengroo, Krishan Kumar Saini, Saumya Ranjan Satrusal, Muqtada Ali Khan, Priyank Chaturvedi, Abhipsa Sinha, Sanjeev Meena, and Anup Kumar Singh.
Breast cancer is the most common malignancy in women, worldwide, having 2.09 million new cases diagnosed in 2018 with 0.6 million deaths. According to some epidemiological data, TNBC occurs mostly in pre-menopausal young women under 40 years old, accounting for approximately 15-20 per cent of all breast cancer patients. It is highly invasive and poses a severe threat.
Also read: After breast cancer: 5 ways to improve your chances of living longer
“TNBC has a poor prognosis and adverse clinical outcomes among all breast cancer subtypes as there is no available targeted therapy,” write researchers.
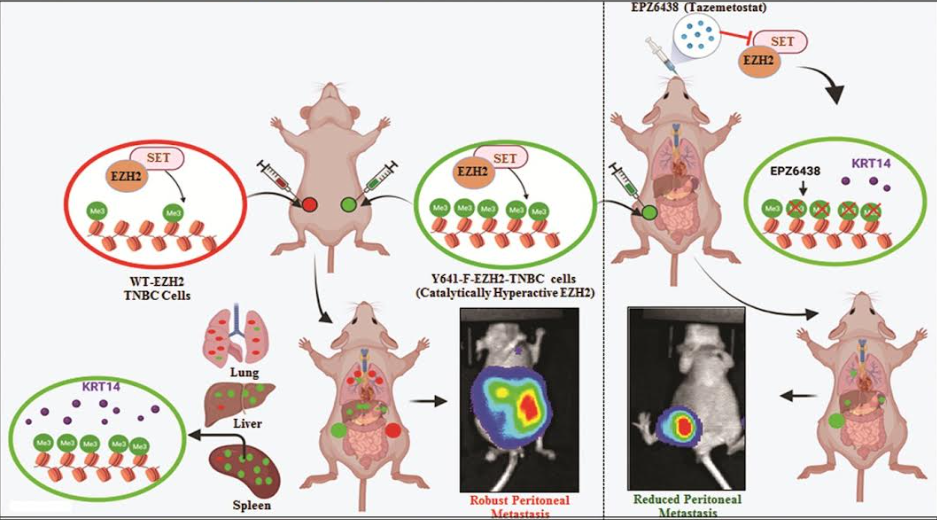
They have discovered that selective hyper-activation of functional EZH2 (Enhancer of zeste homolog 2) over NC-EZH2 (Non-Canonical EZH2) alters TNBC metastatic landscape and fosters its peritoneal metastasis, particularly splenic. The process, further, significantly reduces TNBC migration, invasion, and peritoneal metastasis (spread of cancer from other organs).
The activity of EZH2 has been linked with the development of a tumour/s. It is thought to block the expression of specific tumour suppressors. In cancer, EZH2 functions to promote self-renewal and has been shown to be essential for the tumour-initiating cell (TIC) phenotype in breast cancer.
The team screened samples from the biorepository of the Rajiv Gandhi Cancer Institute and Research Centre, New Delhi for TNBC tumour. Preclinical findings suggest a rationale for targeting TNBC with EZH2 inhibitors. New treatment modalities can be further developed to target TNBC based on these findings. The research study has been published in Nature Communications.A

