
- Home
- News
- Analysis
- States
- Perspective
- Videos
- Education
- Entertainment
- Elections
- World Cup 2023
- Features
- Health
- Business
- Series
- Economy Series
- Earth Day
- Kashmir’s Frozen Turbulence
- India@75
- The legend of Ramjanmabhoomi
- Liberalisation@30
- How to tame a dragon
- Celebrating biodiversity
- Farm Matters
- 50 days of solitude
- Bringing Migrants Home
- Budget 2020
- Jharkhand Votes
- The Federal Investigates
- The Federal Impact
- Vanishing Sand
- Gandhi @ 150
- Andhra Today
- Field report
- Operation Gulmarg
- Pandemic @1 Mn in India
- The Federal Year-End
- The Zero Year
- Premium
- Science
- Brand studio
- Home
- NewsNews
- Analysis
- StatesStates
- PerspectivePerspective
- VideosVideos
- Entertainment
- ElectionsElections
- Sports
- Loading...
Sports - Features
- BusinessBusiness
- Premium
- Loading...
Premium
The importance of vaccine cold chain in immunisation drive
To ensure the quality is maintained throughout, vaccines are transported under refrigeration in a process called Vaccine Cold Chain.

Vaccines travel far and wide from its manufacturing point till it is made available to all. Several precautions are taken to preserve vaccine quality during transport and distribution so that what the recipient gets is what is manufactured. If optimal conditions are not met, vaccines can degrade and become useless, defeating the very purpose of making the vaccine. To ensure the quality...
Vaccines travel far and wide from its manufacturing point till it is made available to all. Several precautions are taken to preserve vaccine quality during transport and distribution so that what the recipient gets is what is manufactured.
If optimal conditions are not met, vaccines can degrade and become useless, defeating the very purpose of making the vaccine.
To ensure the quality is maintained throughout, vaccines are transported under refrigeration. Vaccine Cold Chain is the term used for the long chain of stringent storage, transport, and distribution network of vaccines.
The efficacy of the vaccine cold chain impacts an immunisation drive’s success as any snags due to malfunction of equipment or human errors will severely compromise the vaccine potency.
Owing to the stringency, the World Health Organization (WHO) has mandated a global set of vaccine cold chain protocols. For example, Covid-19 vaccines, Covishield and Covaxin must be stored between 2 and 8 degrees Celsius at all times throughout the distribution chain while the Pfizer and Moderna vaccines require ultra-cold temperatures of -60 to -70 deg C.
Why refrigeration is necessary
When vaccines are injected into the human body cells, they trigger the immune system, which produces antibodies against the pathogen. When there is an invasion by a disease-causing agent, the body readily summons the antibodies to fight them and prevent a full-blown infection.
Vaccines are made from weakened or genetically engineered pathogens, or in other words, they are biological products. Due to this, they are sensitive to temperature.
Manufacturers add adjuvants (chemicals, usually special aluminium salts) to aid immune response and stabilise the vaccine. Adjuvants trigger an inflamed condition in body cells, which in turn produce more antibodies. However, adjuvants degrade quickly under prolonged exposure to temperature fluctuations. In other words, whenever the vaccine is exposed to heat-cold or freezing-thawing cycles, it begins to coagulate and form sediments.
To cite an example, studies performed on the Tetanus vaccine indicate that the vaccine completely degrades within a few hours when kept at 60 deg C whereas when exposed to freezing-thawing cycles, the damage was more pronounced, as the proteins in the vaccine began to collapse and unfold.
Therefore, optimal storage temperatures (as specified by the manufacturer) are imperative to retain the vaccine potency till the last outreach point.
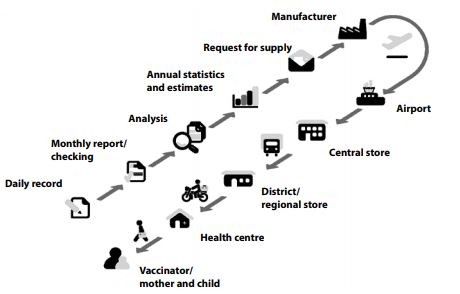
Cold chain components
WHO recommends that all nations and local vaccine regulatory bodies strictly adhere to their Performance Quality Safety catalogue. It has guidelines on the type of equipment, such as the type of refrigerators to be used and other components of the vaccine cold chain. Also, they specify methods and procedures for maintaining storage and transport equipment.
WHO mandates vaccine quality checks (by a method called the Shake Test) at each nodal point, monitoring of equipment maintenance, and ensuring adequately trained vaccinators at the peripheral stage.
Storage categories
Different vaccines call for different storage temperatures. WHO categorises vaccines in six groups labelled A to F. Group A is the most sensitive to heat while group F is the least sensitive. For instance, oral polio vaccine belongs to group A while Hepatitis vaccine to Group F. Group D vaccines (such as Covishield and Covaxin) are the mid-range vaccines with a storage temperature of 2-8 deg C.
Some vaccines should never be frozen, even when transporting them in frigid zones. Also, some vaccines are sensitive to light. Such vaccines come in dark vials and are stored in special dark boxes during transportation to protect them from light damage.
Cold chain points
There are three levels in the cold chain distribution: Primary, Intermediate, and Peripheral.
Usually, the primary and intermediate levels comprise the urban and semi-urban regions with optimal storage facilities like deep freezers, walk-in coolers, cold boxes, and refrigerated trucks for transportation. In contrast, the peripheral level encompasses the last-mile zones, Primary health centres and vaccination centres, including rural, remote and inaccessible regions.
The cold chain must ensure uniformity throughout these levels and take measures to comply with the protocols.
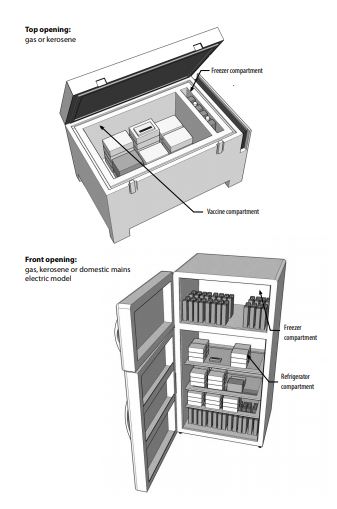
Why not domestic refrigerators for vaccines?
WHO does not recommend using domestic refrigerators to store vaccines because when there is a power cut, these refrigerators fail to maintain the storage temperature for more than an hour or two.
On the other hand, medical refrigerators have special coolants and temperature regulating thermostats and these must be used. These refrigerators can store 20,000-40,000 vials. Also, alternate power backup must be ensured in these places.
Cold chain challenges
The cold chain often becomes vulnerable at the peripheral level and is severely handicapped when vaccines have to reach far-flung and remote regions. In such zones,
- Electricity is in short supply with periods of prolonged power cuts. Setting up alternate power sources is also a challenge.
- Poor road conditions and lack of refrigerated transport are frequently seen. Often vaccines are transported on bicycles and two-wheelers where the vaccine cold-box may be inadequate to last the journey.
- There is a shortage or lack of trained personnel or vaccinators.
In a 2013 study Manoj Muhrekar et al. examined the vaccine’s quality along the distribution chain across 10 Indian states. They found that many incidents occurred where the vaccines were exposed to suboptimal conditions, severely compromising the vaccines’ quality. However, while some zones immediately detected and corrected the shortfall, many others could not, leading to the vaccines’ wastage.
Will Covid vaccination choke ongoing immunisation programmes?
India has one of the largest immunisation programmes globally in terms of cold chain equipment involved. Ahead of the Covid vaccination drive, the health ministry declared to increase the number of equipment to meet the demand of inoculating the entire population.
However, an earlier WHO report states that India still lags in global standards in equipment repairs and maintenance, handling and storage efficacy, pharmacovigilance, and adequate training of vaccinators.
Another area of concern is the overlap of the Covid vaccine drive with other universal immunisation programmes. For example, in Karnataka, polio, and Mission Indradhanush immunisations are rolling out. According to a Deccan Herald report, ‘as many as 64 lakh children missed regular vaccines due to the pandemic and await their shots.
Authorities are facing a huge challenge to coordinate vaccinators for these programs. It is quite probable the state may choose to suspend COVID-19 vaccination during this period.’
Other states may encounter different difficulties. How these factors impact the humungous task that lies ahead remains to be seen.

