
- Home
- News
- Analysis
- States
- Perspective
- Videos
- Education
- Entertainment
- Elections
- World Cup 2023
- Features
- Health
- Business
- Series
- Economy Series
- Earth Day
- Kashmir’s Frozen Turbulence
- India@75
- The legend of Ramjanmabhoomi
- Liberalisation@30
- How to tame a dragon
- Celebrating biodiversity
- Farm Matters
- 50 days of solitude
- Bringing Migrants Home
- Budget 2020
- Jharkhand Votes
- The Federal Investigates
- The Federal Impact
- Vanishing Sand
- Gandhi @ 150
- Andhra Today
- Field report
- Operation Gulmarg
- Pandemic @1 Mn in India
- The Federal Year-End
- The Zero Year
- Premium
- Science
- Brand studio
- Home
- NewsNews
- Analysis
- StatesStates
- PerspectivePerspective
- VideosVideos
- Entertainment
- ElectionsElections
- Sports
- Loading...
Sports - Features
- BusinessBusiness
- Premium
- Loading...
Premium
How scientists propose to fight drug-resistant bacteria, give antibiotics a miss

When antibiotics were introduced in the 1940s, they were considered a boon as they saved millions of lives from fatal infections like sepsis. However, antibiotics have been overused, misused, and even abused over the decades. The indiscriminate use has landed us on the brink of a silent pandemic: the bacteria have developed a resistance to the drugs, and several common bacterial infections...
When antibiotics were introduced in the 1940s, they were considered a boon as they saved millions of lives from fatal infections like sepsis. However, antibiotics have been overused, misused, and even abused over the decades. The indiscriminate use has landed us on the brink of a silent pandemic: the bacteria have developed a resistance to the drugs, and several common bacterial infections like respiratory, gut, skin, and soft tissue infections and surgical and diabetic wound infections are not yielding to even potent new antibiotics.
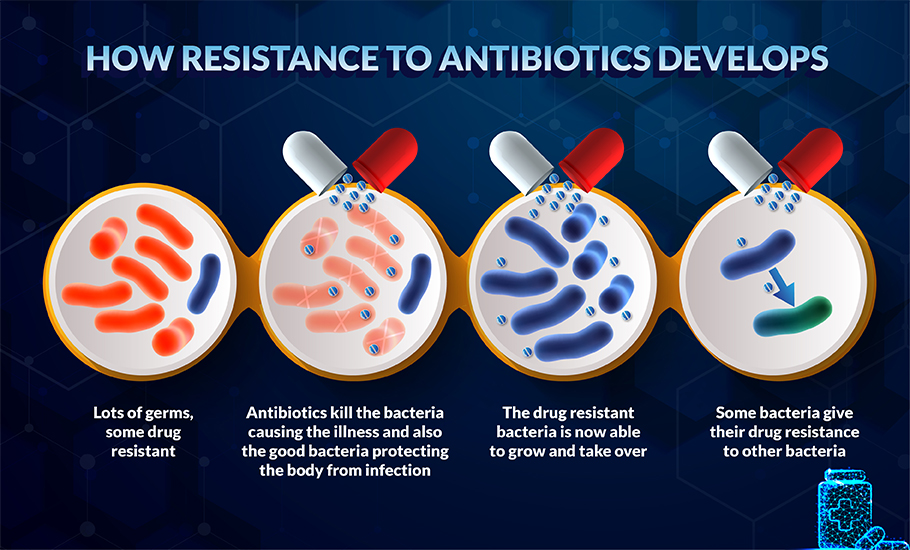
A study published in The Lancet shows that over 1.2 million people died due to drug-resistant bacterial infections in 2019 – the toll far exceeding those due to HIV or malaria. COVID-19 worsened the situation as, in many cases, antibiotics were used irrationally out of fear or ignorance. The global health threat is more in developing nations as they lack proper regulations on the sale of antibiotics as many are available without a prescription. These factors have made people more susceptible to bacterial infections.
Advantage bacteria
Some antibiotics weaken the bacteria’s cell wall (outer covering of the cell); others disturb critical cell functions like metabolism (absorbing and using essential nutrients), multiplying mechanisms, or ribosome activity (the protein manufacturing unit).
Each antibiotic has a targeted action, works on specific types of bacteria, and is therefore ineffective on others – a reason why we have so many types of antibiotics.
Overexposure to these drugs has helped the bacteria turn the tables on us. Studies show that the bacteria have developed an arsenal of special strategies that nullify the effect of antibiotics. Some have modified the way they manufacture their cell proteins, and others have tweaked their genes. Yet others have evolved into micro miners. For example, bacteria get their supply of essential minerals like iron and zinc from our cells. Our immune system reacts to the invasion with nutritional immunity — blocking the availability of the minerals by storing them in particular proteins like haemoglobin. In retaliation, the bacteria release siderophores —proteins with a high iron affinity. Siderophores latch on to haemoglobin, scavenge the minerals, and store them in their cells.
By such evolutionary strategies, highly infectious bacteria described as the ESKAPE group have developed resistance to multiple drugs. ESKAPE is an acronym for – Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.
Emerging strategies
Advanced technologies such as genetic and molecular engineering and nanotechnology offer hope in our battle with the bugs. Scientists are fervently designing drug-free techniques and targeted (inside the bacteria cell) drug-delivery methods for a solution.
These techniques minimise the amount of antibiotics and the side effects. Also, as they can target multiple cell functions in the bacteria, the treatment can be more effective.
AMPs and STAMPs – multi-hit mechanisms
All organisms produce a class of peptides. Peptides are a small chain of 10-50 amino acids (building blocks of proteins). Several peptides are known to have antimicrobial properties and are called Anti-Microbial Peptides (AMPs). Bacteria also produce these peptides to keep other preying bacteria at bay.
AMPs are like small charged wires – their chemical structure gives them an overall positive charge. In contrast, bacteria have an overall negative charge on their cell wall.
When AMPs engage with bacteria, they act like zappers and neutralise the bacteria’s charge. This action creates cracks in the cell membrane, which compromises the cell’s stability and weakens the organism.
AMPs were first isolated in the 1980s. Researchers are actively looking into synthesising such peptides for targeted therapy of bacterial infections. A global AMP database called APD31 stores all the information on AMPs and reportedly has 3,240 AMPs as of August 24, 2020, to benefit research.
In addition, scientists are also designing Special Target AMPs or STAMPs. They have multiple actions: rupture the cell wall, enter the cell and release drugs inside the bacteria’s cell to disrupt specific functions.
Studies indicate that although bacteria can develop resistance to AMPS, evading multi-target AMPs can be difficult as they have to fight multiple invasions into their cells.
Although AMPs offer a natural way to combat bacterial infections, they cannot be given as oral medicine or injected into the veins because enzymes in the body degrade them quickly. However, they work well as sprays and ointments; a few approved AMPs are already in use.
Where light and nanotechnology do the trick
Studies have shown that metal nanoparticles of silver and gold can inhibit the ESKAPE group of pathogens. Primarily, they disrupt the cell activities by disturbing the ion concentration around the bacteria and destabilising it. (Nanoparticles are tiny substances whose dimension is 1/1000000000 of a metre, or a lakh time thinner than a strand of hair).
Many bacteria attach to the surfaces they infect and secrete substances around them, like a mesh. The thick mesh called biofilm embeds within the surface cells forming a shield for the bacteria. Colonies of bacteria reside inside these biofilms; common examples are dental plaque and skin infections. Biofilms cause chronic conditions.

Biofilms are highly resistant to multiple drugs. Antibiotics and immune cells cannot penetrate the barrier. Hence the infections persist stubbornly.
In such cases, two therapies that use a mild type of radiation combined with nanotechnology are showing encouraging results: Antimicrobial Photodynamic Therapy (aPDT) and Photothermal Therapy (aPTT). Both methods use nanoparticles called photosensitizers that are sensitive to particular light radiation and are targeted at the infection site.
In aPDT, photosensitizers absorb the light energy and become excited (high energetic) molecules. They are highly reactive to biomolecules like proteins and cell components. In the presence of oxygen, the reaction releases what are called Reactive Oxygen Species (ROS) and free radicals. These free radicals are toxic; they overwhelm and choke the bacteria, destroying them. Multi-action nano-photosensitizers disrupt biofilms, reach the bacteria and destroy the colony.
It is well known that heat kills bacteria, and Antimicrobial Photo Thermal therapy (aPTT) uses this property. In this method, metal and metal oxide nano-photosensitizers are excited by infrared radiation. They absorb the light and jump to a higher energy state. At the infection site, they not only generate free radicals but also dissipate heat. Hyperthermia (excess heat) kills the bacteria.
Accurately targeting the infection site is the pressing challenge in these techniques.
Novel finds
Recently, researchers at the Institute of Nanoscience and Technology, Mohali, discovered that some strains of ESKAPE group bacteria were storing iron and zinc in the form of magnetic nanoparticles in their cells. When they exposed the bacteria to an external alternating magnetic field, the nanoparticles scurried back and forth rapidly (called Brownian motion). The rapid movement dissipated heat from the nanoparticles, inducing hyperthermia inside the cell. They could destroy 70-80 per cent of the bacteria in infected tissue samples by this method. They are now exploring prototype device options to treat diabetic wound infections by this method.
In another study, UK scientists have identified a master protein called DsbA in infectious bacteria. They found that DsbA is responsible for folding and shaping the drug-resistant proteins in the bacteria. They are finding ways to inhibit the function of DsbA.
These findings and technologies together can help us avoid the use of antibiotics to fight the silent pandemic we have unleashed.

