Explained: What are CRISPR-Cas9 genetic scissors?

The 2020 Nobel Prize in Chemistry has been awarded to Emmanuelle Charpentier and Jennifer A Doudna for “the development of a method for genome editing”. Prof Charpentier is currently the Director at the Max Planck Institute for Infection Biology, Berlin and Prof. Doudna is Professor at the Department of Molecular and Cell Biology at the University of California.
The award was given for their work which resulted in the discovery of the molecular mechanisms of the bacterial CRISPR-Cas9 immune system and repurposing it into a tool for genome editing.
Related news: Chemistry Nobel awarded to Emmanuelle Charpentier, Jennifer A Doudna
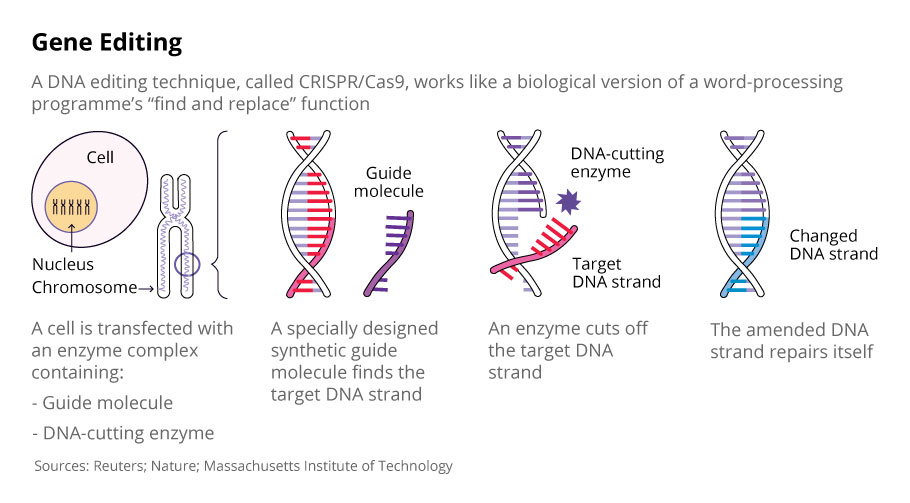
The CRISPR-Cas9 system consists of two molecules that make an edit in the DNA.
1) Cas9 – An enzyme which functions as a pair of ‘molecular scissors’. It has the ability to cut the two strands (sense and anti-sense) of DNA at a specific location in the genome.
2) guide RNA (gRNA) – A short (20 bases or so) piece of pre-designed RNA sequence located within a longer RNA scaffold. The gRNA guides the Cas9 to the planned part of the genome ensuring the right place is cut. The gRNA has bases which are complementary to the target sequence in the DNA like a very specific lego piece. This in theory ensures that the gRNA binds only to the desired sequence and not somewhere off-target.
The Cas9 scissor enzyme is guided to the desired location and snips across both strands of the DNA. Once this happens the cellular mechanism understand that the DNA is damaged so initiates a repair mechanism. At this juncture it is possible to use this opportunity to introduce changes to the genes of their design.
One of the most essential parts of molecular biology is to understand the function of a particular gene. For many years the only way to study that was by making a small change (mutation) to the gene and see what effect it has on the genome or even the whole organism. For example a tiny change in the Dystrophin gene, depending on where and how the mutation is could lead to a mild form of Dystrophy called Becker’s Muscular Dystrophy (BMD) or a fatal version called Duchenne Muscular Dystrophy (DMD).
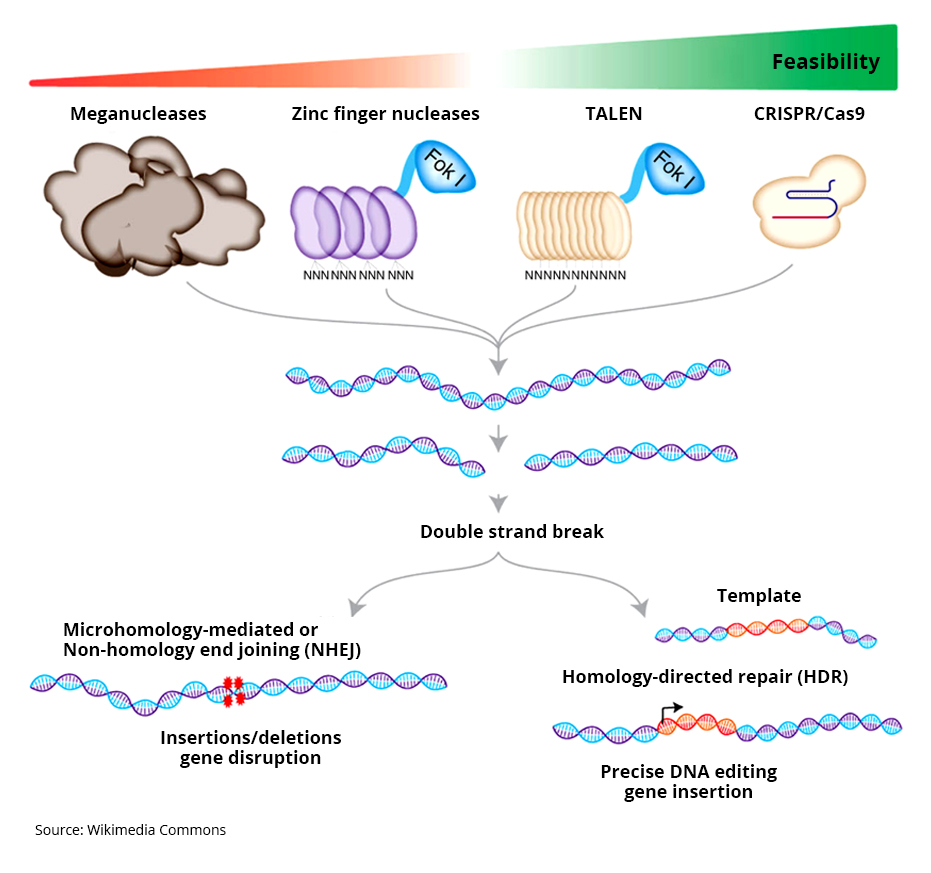
For a long time the only way molecular biologists could study this was by using chemical or radiation to cause these mutation, but a major drawback was that there is no way to control the targeting of these mutation events. There were specific gene targeting methods but were not reliable, were very time consuming and extremely expensive. The CRISPR-Cas9 system is by far the fastest and more inexpensive method.
Francisco Mojica faculty of the University of Alicante was the first person to report the CRIPR locus in 1993. Earlier Japanese researcher Yoshizumi Ishino had described part of it in 1987 but it was Mojica who was able to completely study the sequence and their function. Mojica and Ruud Jansen of Utrecht University coined the term CRISPR which was an acronym for ‘Clustered
Regularly Interspaced Short Palindromic Repeats’. The enzyme adds bits of viral DNA to its own genome to guide the Cas proteins. These small bits of DNA are the what name CRISPR defines. In 2003 he wrote a paper in which suggested that CRISPR was an innate immune system in Bacteria but 4 major scientific journals rejected it and it was only published two years later. Mojica wrote about a protein named Cas9 found in the Streptococcus bacterial “CRISPR” immune system that cooperates with guide RNA and works like scissors snipping at a gene sequence. The protein’s role in the immune system is to attack the DNA of viruses which infect the bacteria and slices it up effectively stopping it from causing further damage to the bacterium.
In 2011, Charpentier demonstrated that a small RNA called trans-activating crispr RNA (tracrRNA) is essential for the maturation of crispr RNA (crRNA) and in the process uncovered a novel mechanism for the maturation of a non-coding RNA which is pivotal in the function of CRISPR/Cas9. Charpentier and Doudna met at a conference and began a collaboration which resulted in a paper published in Science in August 2012. In this they put forth the idea that Cas9 could be used to make cuts in any DNA sequence desired.
Another researcher who is part of this revolution is Feng Zhang who is a biochemist at the Broad Institute of MIT. His lab began work to harness and optimize the CRISPR system to work in human cells in early 2011 shown that CRISPR-Cas9 could edit genes in cultured human cells a few months after Doudna and Charpentier published their method.
CRISPR/Cas9 is already widely used for scientific research and has the potential to transform medicine, enabling us to not only treat but also prevent many diseases. The system has already been used for some revolutionary work in molecular biology such as fingerprinting cells and logging what happens inside them to directing evolution.
One interesting avenue is the creation of gene drives using this system. This fast and cheap method of editing DNA is going to be a major driving force in Molecular Biology for decades to come and we are now standing at the cusp of personalized medicine becoming a normal occurrence. As of now CRISPR-Cas9 editing done in somatic cells is completely legal and ethical and as long we keep away from germ-line mutations this system shows excellent promise for the world of Genetics.


