No intermixing of COVID-19 vaccines, say Centre's guidelines

Ahead of the COVID-19 vaccine rollout in the country — billed as the world’s biggest such exercise — the Ministry of Health & Family Welfare has sent comprehensive dos and don’ts to the states and UTs for both the vaccines, Covishield and Covaxin, listing dosage, cold chain storage requirements and minor adverse event following immunization.
The directives very clearly state that there should not be any intermixing of vaccines – the second dose should be of the same vaccine of which the first dose was administered.
According to the fact sheet, vaccination is allowed only for those who are above the age of 18 years. Women who are pregnant or not sure of their pregnancy and lactating mothers should not receive the vaccine, which is meant for people who are 18 years old or above.
Also read: 3 lakh health workers to get inoculated on day 1 of COVID vaccination drive
Some of the other salient points of the guidelines are:
* The vaccine should be given with ‘caution’ to persons with a history of any bleeding or coagulation disorder/platelet disorder, clotting factor deficiency, or coagulopathy.
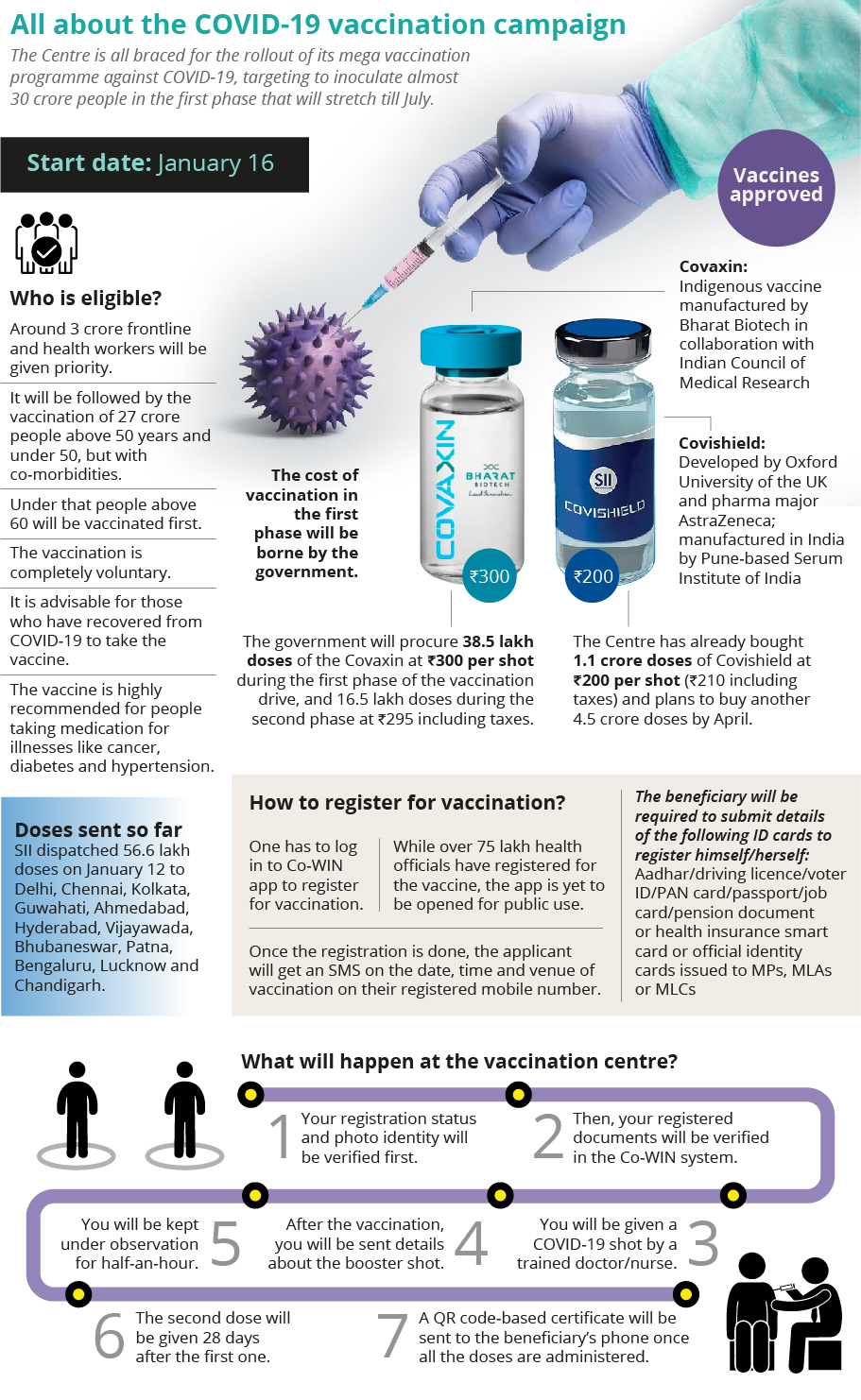
* Vaccinators have to store both the vaccines at plus 2 degree C to plus 8 degree C and protect them from light. They should discard a sample if found frozen.
* The vaccines cannot be given to those with a history of an allergic reaction to a previous dose of a Covid-19 vaccine (those who have taken a dose in a country where vaccination has already started).
* People who show an immediate or delayed onset of an allergic reaction to vaccines or injectable therapies, pharmaceutical products, and food items.
* Vaccination has to be put off for four to eight weeks after recovery from certain conditions such as in those showing active symptoms of SARS-CoV-2 infection, COVID patients who have been given anti-SARS-CoV-2 monoclonal antibodies or convalescent plasma and those acutely unwell and hospitalized.
Also read: After frontline vaccination, govt may allow Covishield, Covaxin export
The government has said adequate doses of Covishield and Covaxin have been delivered to all states and UTs. As many as 3,006 session sites across all states and UTs will be virtually connected during the launch and around 100 beneficiaries will be vaccinated at each session site on the first day, a government statement said.
On January 16, the Prime Minister will also launch the government’s CoWIN (Covid Vaccine Intelligence Network) app, a digital platform built for real-time monitoring of the vaccine delivery and distribution.
The guidelines say healthcare workers (those registered in Co-WIN to be vaccinated) will include not only doctors, nurses but also nursing orderlies, safai karamcharis, ambulance drivers, and would be from a mixed age group, including above 50 years.